The
Coral Health and Disease Consortium:
New Information on
Coral Disease
During
January 22-25, 2002, I was an invited participant in the
first official workshop, "Coral Health and Disease:
Developing a National Research Plan," of the Coral
Disease and Health Consortium (CDHC). The CDHC was created
in response to the Coral Reef Task Force's National Action
Plan with the expressed purpose of organizing and coordinating
scientific resources to focus specifically on coral health
issues (Woodley 2002).
The workshop participants
were experts in varied disciplines, and included those involved
in coral disease research, as well as people from veterinary
medicine, the health profession, and biotechnology. This
cross section of disciplines was an intentional effort to
enlist techniques and ideas from various fields to help
accelerate the slow progression of advances in the study
of coral disease.
Before delving into the
proceedings and results of the workshop, I will briefly
make a few comments regarding this event - several findings
that came as somewhat of a surprise to me. Since the mid-to
late-1990's, I have been suggesting that coral disease is
likely to have a strong correlation to stress, and that
coral immunity should be looked at in terms of its relation
to coral disease (Borneman and Lowrie 1998a, 1998b). My
initial thoughts were met with some controversy, yet I felt
- and feel - strongly enough about it that I have made it
the focus of my doctoral dissertation work. It came as a
quite a pleasant surprise to see that a focus of this workshop
was towards beginning to look at coral immunity and the
relation of stress to coral disease. In fact, our working
group (one of only four) was designated with the title "Environmental
Factors Affecting Susceptibility and Infectivity."
To say I was thrilled to be part of this group would be
an understatement.
Paradoxically, this same
workshop gave me some considerable measure of pause regarding
my thoughts on the roles of pathogens in coral disease.
For an equally long period of time, I have been adamantly
saying that the number of people suggesting that "mystery
pathogens and bacteria" seem to be the root of all
coral disease were probably mistaken. I feel more strongly
than ever, and based largely on the amount of supporting
research in this area (Herndl and Velimirov 1986, Koh 1997,
Paul et al. 1986, Rowher et al. 2001, Santavy 1995, Shasar
1994), that bacteria and corals are associatively linked
and in most cases have benign or even beneficial relationships.
However, I am also more convinced that bacterial pathogens
may be more prevalent or have a greater role in disease
than I had previously wanted to admit. I hope that this
view will be clarified over the following article.
The White Papers
Prior to the workshop, and
included in the materials given to us, were a number of
papers by coral disease researchers that summarized current
status in the field or that presented new information that
was either currently in press or being submitted for publication.
Thus, we got a "sneak preview" of some exciting
work. Unfortunately, I cannot relate the exact nature of
some of this material until it has been published. There
is plenty, though, that I can relate.
Coral Histology
Esther Peters, one of the
pre-eminent coral pathologists, presented information summarizing
the use and progress of histological techniques used to
study coral disease. Histology is the study of tissues and
their structure function, usually through microscopy. She
is a leader in this area, and in addition to showing some
of the tools currently available to study histology in corals,
she made several important comments. The study of the histopathology
of corals is still in its infancy and those contributions
in the etiology (factors that cause or play a role in the
expression) of diseases will be helped by interdisciplinary
studies, "incorporating information from analytical
chemistry, biochemistry, microbiology, molecular biology,
and physiology, as well as oceanography and ecology, and
new developments in histotechniques." She pointed out
how important histopathology is in understanding coral disease,
and that so much work is need to determine how disease "might
be the result of infections with different pathogens and/or
physical or chemical stressors." (Peters 2002).
The Scope and Management
of Coral Disease
Ernesto Weil presented material
based on his current and upcoming publications discussing
primarily the scope and range of coral diseases throughout
the Caribbean (Weil 2002a, 2002b). He mentioned that coral
disease is found in the Pacific, as well. Much of the study
of coral disease is in the Caribbean, and Ernesto showed
photos of disease events throughout the region. He also
showed photos of new putative coral diseases, many of which
I had also brought to show the group from my own diving
in the region. A real question is, to what extent the signs
of these diseases represent separate phenomena or are simply
different appearances of the same phenomena? Answering this
question is hampered greatly by the difficulty and slow
progress in determining real causes of coral disease (and
of course, one of the very reasons for this workshop).
 |
This
Colpophyllia natans, photographed at the Flower
Garden Banks, had a highly aberrant mottled pattern
on its surface that consisted of both bleached areas
and necrotic areas. This is an undescribed condition
that has been seen elsewhere (Weil pers comm), but
the coral apparently recovered within several months
(Wiseman pers. comm). |
 |
The darkened
rings on this Montastraea faveolata in Puerto
Rico is not normal. The tissue itself looked healthy,
and the cause of this banding is not known or described. |
|
|
The Diplora
strigosa in these photos from the Flower Gardens
were not unusual. Numerous colonies have this unusual
patttern that seems to be a version of the common
hypertrophic growths (tumors) that are evident on
many corals in this area. The cause of this condition
is unknown and undescribed. |
 |
This
ring-type pattern is being seen more commonly in Sidearastrea
siderea, a species well known to display the condition
called Dark Spots Disease. There is no known cause
for either condition, and the rings may be a variation
of the more spotty Dark Spots. However, the consistency
of variation in the signs of the disease, albeit with
some overlap, is remarkable. Photographed at Mona
Island. |
 |
These pale ring
shapes were seen on several species of Diploria
and Colpophyllia around Mona Island. The tissue
appeared healthy, but the coloration was aberrant
enough that we tagged the colonies for future observations.
Apparently, the colonies had recovered their normal
pigmentation several months later (Bruckner pers comm.).
|
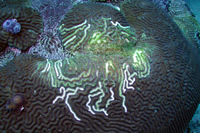 |
This is a disease
known as Ridge Mortality Disease and is only reported
on Diploria sp. Some researchers feel that
it is not a disease, but results from the nipping
actions of damselfish. There are indeed cases where
this is likely to be the case, but damselfish tend
to work in patches and non-linearly along a ridge.
Furthermore, no damsels were seen nesting around this
colony. Photographed at the Flower Garden Banks. |
 |
A band of unknown
origin on Siderastrea siderea. Photogrpahed
at Southwater Caye, Belize. |
|
|
This
type of pattern would be called Patchy Necrosis by
some definitions, but Patchy Necrosis is supposed
to only occur on Acropora palmata. Photographed
in Tavernier Key, Florida. |
 |
A
new outbreak of Patchy Necrosis on A. palmata
has recently been reported following a warm, still
period in Parguera, Puerto Rico. I photographed this
specimen many months earlier than the reported "outbreak"
at the same location. Thse signs resemble "RTN"
in aquarium corals. |
 |
This would
ordinarily be called White Plague on Montastraea
faveolata. However, Plague usually forms a solid
line across the tissue, and across corallites. Here,
the outline follows each polyp exactly. It is unusual
in this regard. Photographed in Cozumel, Mexico. |
Doctor Andrew Bruckner gave
a presentation on his comprehensive (49 pages!) white paper
entitled "Priorities for Effective Management of Coral
Diseases." Andy suggested the following points as being
important in the management of disease in his summary:
| 1) |
an early warning system
to predict and identify disease outbreaks; |
| 2) |
documentation of spatial
distribution and temporal variations of coral
diseases and other syndromes at local to global scales;
|
| 3) |
elucidation of relationships
of environmental stressors, localized
anthropogenic impacts, and widespread phenomena such
as global warming and El Niño on coral health,
disease, degradation and recovery; |
| 4) |
development of standardized
terminology for diseases and other
syndromes through a characterization of the visual appearance,
pathology and etiology, and the development of molecular
probes and other tools to identify and verify diseases
in the field; |
| 5) |
identification of factors
that facilitate the introduction, spread and
transmission of pathogens; |
| 6) |
research on the effects
of disease on coral species and populations,
associated species, and ecosystem structure and function;
and |
| 7) |
implementation of measures
to mitigate disease impacts, including
strategies that reduce anthropogenic stressors responsible
for the proliferation or spread of diseases and the
development of novel techniques to treat affected corals
and improve habitat quality. |
| |
(Bruckner, 2002) |
Laurie Richardson and Richard
Aronson also had a paper outlining the various techniques
being currently used in coral disease, the need for interdisciplinary
action, and the relatively poor state of knowledge that
exists, given the impact and extent of current coral diseases
(Richardson and Aronson 2002). Garriet Smith summarized
his research and progress in isolating coral pathogens and
in determining the extent to which they represent normal
or abnormal microbial flora to coral surfaces in a presentation
titled, "Disease Identification: Technologies."
There was also an excellent presentation by Dr. Jonas Almeida,
titled "Bioinformatics and Coral Disease," on
the use of bioinformatics and neural networks to begin working
with databases to increase relative performance of existing
data and to help detect trends not easily available with
current non-centralized efforts.
Despite the quality and
importance of these papers and presentations, there were
several others that, for me, "stole the show."
I will limit the remainder of this article to discussing
aspects of those talks, and perhaps delve into results of
the workshop in the next article.
Standards for Disease
in Other Fields
Pam Parnell of the Clemson
Veterinary Diagnostic Center presented ideas that were to
become a focal point of the entire workshop in her presentation
titled, "Disease Investigation: The Process."
In particular, her words have great importance to aquarists,
as well. Coral disease research has been somewhat of a reactive
science in that once a disease is noticed, frantic work
is begun to try and uncover the etiology of the disease.
The goals and methods used by the community have little
in common with established practice in other health areas,
and she used her expertise in veterinary medicine to state
the major points. First, in all medicine there is a very
strict and complete language and terminology used to describe
disease. This encompasses sometimes exceedingly intricate
detail in describing lesions and other aspects of the disease.
The language is developed such that everyone involved in
the field can communicate and know exactly what is being
described. No such language exists in the coral disease
field. This stems, in part, from our relative ignorance
about exactly what these things are, but another part is
a lack of cohesiveness among the researching body. Without
being able to describe and articulate what various observers
are seeing, the understanding of epizootics and disease
will remain stifled. This problem is apparent in the terminology
of the diseases where terms such as White Plague, White
Plague Type II, and now White Plague type III exist. Similarly,
Yellow Blotch Disease, Yellow Band Disease, and Yellow Line
Disease may be used to describe the same condition. Arnfried
Antonius, despite having been integral in the description
of recognized "white" diseases (Antonius 1995),
now recognizes this limitation of the nomenclature and lumps
all diseases with unknown etiologies that are characterized
by a white line delineating healthy tissue from denuded
skeleton, as "White Syndromes."
Parnell also discussed the
need for a centralized "center of operations"
or communication center to facilitate the dissemination
of reports, research, findings, databases, reference libraries,
and discussions. Furthermore, she emphasized the need for
a coral "guinea pig," a need also recognized almost
unanimously by the attendees. In other words, a model coral
needs to be established that can be used for both baseline
information (genetics, physiology, etc.) and for disease
studies.
For aquarists, these suggestions
are also very important. In The Coral Forum on Reef Central,
countless requests are made that describe a coral in poor
health. It is very difficult to assess the actual nature
of the problem, syndrome, or disease without being able
to adequately communicate the event in question. In that
regard, I will pass along those core standards to the hobby
in a future publication, perhaps with practical revisions
once they are established, so that we can effectively communicate
within the hobby, as well. Furthermore, our observations,
if properly documented and communicated, can be valuable
additions to the coral disease body of knowledge, in general.
The Use of Biotechnology
Craig Downs is one of the
founders of EnVirtue Biotechnologies. While not expressly
involved with corals, Craig has various assays and technologies
either available or potentially available to make coral
disease work progress much more quickly. Many of the technologies
he discussed in his talk, titled "Abiotic Factors Infecting
Infectivity and Suceptibility," were very similar to
ones being used in other fields for years. Michael Gerdes
and Frank Marini, both Reef Central members, have discussed
this many times with me. Michael works at NIH and astounds
me with the technologies available in human medicine, as
does Frank who works at the MD Anderson Cancer Research
Center. They know the powerful nature of the available tools
and how little they are being used in marine science. Fortunately,
this may be changing. Among the many topics discussed was
the use of conserved areas of the genome to probe for various
stresses and for the identification of putative pathogens.
Of particular interest were assays available for stress
proteins that are nearly, if not totally, conserved among
animals and even bacteria, such as ubiquitin, NADPH oxidase,
heat shock proteins (particularly hsp60), and others. Also
of great interest was the investigation of iron in coral
tissue and coral surfaces. Iron is normally sequestered
by organisms because bacteria need it for growth. It is
an immunoresponsive action. While corals are not vertebrates,
many basic immunological processes are similar, analogous,
or are likely to be found but are as yet undetermined. Iron
sequestration by molecules such as lactoferrin and transferrin,
or analogues, would be easily investigated using existing
methods and would likely be productive. Finally, Craig introduced
another buzzword that many of us were already thinking about
- apoptosis.
Cells die in one of two
ways - necrosis and apoptosis. Necrosis is a passive degenerative
process that entails that destructive enzymatic processes
are involved.
So, what is the significance
of apoptosis? Apoptosis is programmed and induced cellular
suicide. The last few years have witnessed an explosion
of interest and knowledge about apoptosis, the process by
which a cell actively commits suicide. It is now well recognized
that apoptosis is essential in many aspects of normal development
and is required for maintaining tissue homeostasis, such
as the limited lifespan and renewal of white blood cells.
Failure to properly regulate apoptosis can have catastrophic
consequences. Cancer and many diseases (AIDS, Alzheimer's
disease, Parkinson's disease, heart attack, stroke, etc.)
are thought to arise from deregulation of apoptosis. Apoptosis
has emerged as a key biological regulatory mechanism (www.apopnet.com).
In senescent organisms, apoptosis is hard wired into the
genetic code, and numerous apoptosis genes have already
been discovered (bcl-2, bax, bcl-Long, bcl-Short,
caspase (several), and fas). However, apoptosis can
also be triggered by stress, and bacteria are known that
produce toxins and other molecules that can trigger apoptosis.
Several years ago, I argued
that RTN had the appearance of autolysis (Borneman and Lowrie
1998a, 1998b). Autolysis has also been estimated as occurring
with members of the family Xeniidae (Fabricius and Aldeslade
2001), Stylophora pistillata (Muller et al. 1986),
and other corals as described in Borneman and Lowrie (1998a,
1998b). Over a year ago, I looked at an Acropora sp.
from my tank that had contracted "RTN," as part
of a coral histopathology workshop. Esther Peters described
the tissue at the time as a coagulative necrosis. Under
the microscope, the tissue was sloughing in blobs, with
many zooxanthellae still intact within the blobs. There
were no unusual bacteria noticed within the tissue, or external
to the tissue. However, this was but a single sample and
the fixation process could have removed external pathogens.
Similarly, staining and light microscopy may not have been
able to easily resolve intracellular bacteria. I am currently
embedding more specimens with RTN for examination, but this
is not the focus of this finding. The description of apoptosis
is that apoptotic bodies are produced by a process called
blebbing. What this means is that blobs of cellular material
are produced as the cell kills itself. Needless to say,
this will be an area of great interest and work for me in
the coming years.
 |
An
Acropora sp. with RTN, also known as Shut Down
Reaction, currently under study from Houston, Texas. |
 |
Trachyphyllia
geoffroyi with RTN in Houston, Texas. Is this
apoptosis? |
 |
A typical
Shut Down Reaction, also known as RTN, in Galaxea
fascicularis, photographed in Jakarta, Indonesia.
|
The Amazing Case of Vibrio
shiloi
Several years ago, an article
appeared that described the bacteria, Vibrio shiloi,
as causing bleaching in the Mediterranean coral, Oculina
patagonica (Kushmaro 1996). At the time, most people
were of the opinion that the conditions of this were unusual.
It seemed to occur in a single species in a non-coral reef
area. Most researchers were relatively unconcerned. Julian
Sprung spoke vocally about this event in a discussion on
NOAA's coral-list, and it was similarly met with some skepticism
that it could be much of an issue for corals, in general.
To be sure, I was one of them.
However, one could have
heard a pin drop during the elegant and outstanding presentation
by Dr. Eugene Rosenberg of Tel Aviv University (Rosenberg
2002). This man single handedly threw the proverbial monkey
wrench into the coral world that morning. In the years since
the original articles have been published, Rosenberg's team
has not only fulfilled Koch's postulates for this pathogen
in a textbook-like fashion, but has proceeded to describe
the etiology in an extremely impressive manner. I would
urge those interested to read the articles listed below
that relate to this disease.
 |
A wild Acropora
cervicornis with classic Shut Down Reaction, or
RTN, in my aquarium hours after being collected and
shipped from Puerto Rico. |
 |
This Trachyphyllia
geoffroyi is showing signs of a progressive and
spreading-type bleaching. Is this bacterial bleaching?
The coral is currently under study.
|
In short, Vibrio shiloi
is a newly described species of bacteria, related to V.
mediterranei, with an as-yet undetermined reservoir;
that is, it is not known where or if the presence of this
bacteria is normal to the environment, or if it is somehow
just recently showing up to affect the area. It follows
the temperature cycles of the area precisely, and causes
bleaching in warm months followed by recovery as the water
temperature declines.
To infect Oculina patagonica,
V. shiloi forms an adhesion with a ß -galactoside
receptor on the coral surface and is specific for the coral
and the bacteria. One pathogen, one host (although V.
shiloi has infected other corals in laboratory studies).
The temperature at which adhesion occurs is critical, with
adhesion only occurring if the bacteria (and not necessarily
the coral) is grown at the elevated temperature required
to induce the virulence factor. Even more recently, the
receptor was found to exist in the coral mucus, and that
photosynthesis by zooxanthellae is required for the synthesis
and secretion of the receptor. Interestingly, it only takes
120 bacteria to cause an infection, and the bacteria can
reproduce to 109 bacteria/cm3
in five days!! With water cooling below the virulence temperature,
the bacteria die rapidly.
Another fascinating aspect
is that the virulence factor is, in fact, a housekeeping
gene (a normal metabolic gene). Superoxide dismutase (SOD)
is produced both by corals and V. shiloi at high
temperatures. Mutants of V. shiloi which lack the
gene to make SOD, adhere to the coral, penetrate it, and
die from the oxygen radicals produced by the photosynthesizing
zooxanthellae. It may be that the zooxanthellae have a function
in protecting corals from infection.
Another virulence factor
of V. shiloi is a type of extracellular proline rich
toxin that can inhibit photosynthesis of zooxanthellae by
forming a membrane channel that allows NH3 to pass, changing
the pH gradient that exists across the algal membrane, and
blocking photosynthesis. It, and other as yet uncharacterized
high molecular weight toxins, then bleach and lyse the algal
cells. The levels of toxin production are also correlated
with the high temperature.
The reader may ask the same
question that has occurred before, and was described above.
So what? It's a Vibrio that is found not on coral
reefs, but is specific to one coral species that we don't
keep and will likely never see. The implications are certainly
interesting, but what does it mean to tropical corals? Rosenberg
had an answer to this, too. Knowing the skepticism that
existed in the community, he has recently gone into the
Indian Ocean and the Red Sea and looked at bleached Pocillopora
damicornis. Is everyone ready?
A new species of bacteria,
Vibrio corallyticus, was consistently found in the
tissues of the bleached Pocillopora at a level that
already fulfills the first of Koch's postulates. The virulence
is even more amazing. At 23° C, there are no visible
signs of disease. At 25° C, bleaching occurs. At 27°
C, there is rapid tissue lysis. A virulence factor is being
produced by this bacteria that correlates extremely well
with the temperatures commonly cited as causing coral bleaching.
Furthermore, Rosenberg describes the bleaching as spreading;
a characteristic seen all too often by both field observers
and aquarists.
The implications of Rosenberg's
work are almost indescribable. He is of the opinion that
probably all bleaching is caused by bacteria. Unfortunately,
there are many studies where bleaching has been caused by
low temperature, UV radiation, darkness, chemicals, etc.
(see Borneman 2002). However, the importance of looking
at bleaching in an entirely new light is now at hand. It
has often been questioned why corals in the wild would bleach
with only a 1-2° C temperature change when other areas
(including tanks) routinely experience far greater vacillations
without any bleaching incidence. The fact that virulence
can be expressed with this small temperature increase makes
such accounts explainable. Furthermore, with temperatures
in the oceans having warmed over the past fifty years, and
with bleaching events being more common in recent years,
the existence of bacterial bleaching under such temperature
increases may explain not only the increased incidence of
bleaching, but also explain why mortality is so common in
some bleaching events while recovery happens in others.
As an example, if corals
have been growing in water averaging 26° C, more or
less, for the past several thousand years, and over the
past fifty years the temperatures in the water are now 27°
C. Virulence of a microbe is expressed at 28° C to cause
bleaching. Now, it only takes a 1° C change to cause
virulence genes to be turned on and cause bleaching, and
this occurs much more frequently than the 2° C change
that it took previously. Furthermore, if the water temperature
gets to 29° C, it may not be that the corals have exceeded
their upper thermal limit, but that virulence genes that
cause tissue lysis have been expressed.
Several points regarding
this work should be made, however. First, Oculina patagonica
is a facultatively zooxanthellate temperate to sub-tropical
coral. It is not from coral reefs, and as far as we know,
neither is the Vibrio that causes a problem. The
results with a bleaching Vibrio may still be relatively
unique to this coral and bacterium. Second, the results
involving P. damicornis and V. corallyticus
are in their infancy. The degree to which bacteria play
a role in any other events is a long way away, and no conclusions
should be drawn at this point regarding other similar events.
The water temperatures were low compared to most reef areas
and other factors (both biotic and abiotic) have not yet
been fully considered in this finding. The potential implications
are what are notable.
The major questions remaining
to be answered are many. First, to what extent are bacteria
involved in bleaching events? What is the variation in virulent
species or strains? Are these normal microbial flora that
are heat-activated opportunists? Are they new to the environment?
What is their reservoir and why are they now being expressed?
Are there immune mechanisms that can deal with these pathogens,
and to what degree do various stressors act in the expression
of infectivity and susceptibility?
I have recently prepared
samples of a Trachyphyllia geoffroyi with spreading
signs of bleaching, and of a foliose Montipora sp.
with similar signs from another aquarium. This latter case
was especially intriguing as another colony of a different
species of Montipora (M. digitata) had "caught"
the spread of bleaching. No other corals in the tank were
bleached. Rest assured I will report what I find at a future
date.
 |
A Stylophora
pistillata from a store in Houston, Texas displaying
a classic white band-type disease. The coral is currently
under study. Interestingly, the band line stopped
immediately upon the coral’s placement in my
aquarium (after sampling the tissue) and is recovering. |
 |
A Hydnophora
sp. from a store in Houston Texas, also with a white
syndrome. Here, the tissue was slowly receding, but
the tissue was actually free at the disease line margin
and appeared to be released from its skeleton. |
As a final note to this
incredible tale, and as if the reader has not had enough
already, Rosenberg also found that Oculina in shallow
water, even in high temperature and exposed to V. shiloi,
rarely bleached. They found that UV radiation acted as an
effective sterilizer for V. shiloi on the coral surface!
To be continued......
|
If you have any questions about this article, please visit my author
forum on Reef Central.
|
|
References
Antonius, A. 1995. Coral diseases as indicators
of reef health: field methods.
Proceedings of the 2nd European Regional Meeting, ISRS, Publ
Serv Geol Lux. XXIX: 231-235.
Borneman, E. H. and Lowrie J.. 1998. The
immune response of corals. Part
1: The invertebrate immune system. Aquarium
Net. Summer issue.
Borneman, E. H. and Lowrie J. 1998. The
immune response of corals. Part
2: Models for “RTN.” Aquarium
Net. Summer issue.
Bruckner, A. W. 2002. Priorities for Effective
Management of Coral
Diseases. Prepared for Workshop Coral Health and Disease:
Developing a National Research Plan Coral Health and Disease
Consortium. Charleston, South Carolina, January 22-25, 2002.
Fabricius, K, and Alderslade P. 2001. Soft
Corals and Sea Fans. AIMS,
Townsville: 53.
Herndl, GJ and Velimirov, B 1986. Microheterotrophic
utilization of mucus released by the Mediterranean coral Cladocora
cespitosa. Mar Biol 90: 363-369
Koh, E.G.L. 1997. Do scleractinian corals
engage in chemical warfare against microbes? J Chem Ecol.23:
379-98.
Muller, W. E.G., 1984. Intraspecific recognition
system in scleractinian corals: morphological and cytochemcial
description of the autolysis mechanism. J Hist Cyto 32: 285-8.
Paul, J.H., DeFlaun, M, and Jeffrey, W.
H.. 1986. Elevated levels of microbial activity in the coral
surface microlayer. Mar Ecol Prog Ser 33: 29-40.
Peters, E. C. 2002. Coral disease diagnostics:
histopathology. Prepared for Workshop Coral Health and Disease:
Developing a National Research Plan Coral Health and Disease
Consortium. Charleston, South Carolina, January 22-25, 2002.
Richardson, L.L., and Precht R.B. 2002.
Infectious diseases of reef corals. Proc 9th Int Coral Reef
Symp, Bali. In press.
Rowher, F., Breitbart, M., Jara, J., Azam,
F, and Knowlton, N. 2001. Diversity of bacteria associated
with the Caribbean coral Montastraea franksi. Coral
Reefs 20: 85-91
Rosenberg, E. 2002. The Oculina patagonica-Vibrio
shiloi model system of coral bleaching. Prepared for Workshop
Coral Health and Disease: Developing a National Research Plan
Coral Health and Disease Consortium. Charleston, South Carolina,
January 22-25, 2002.
Santavy, D. L. 1995. The diversity of microorganisms
associated with marine invertebrates and their roles in the
maintenance of ecosystems Cab International, Wallingford:
211-229
Shasar, N, Cohen, Y, Loya, Y, and Sar,
N. 1994. Nitrogen fixation (acetylene reduction) in stony
corals: evidence for coral-bacteria interactions. Mar Ecol
Prog Ser 111: 259-264.
Weil, E. 2002. Coral disease epizootiology:
status and research needs. Prepared for Workshop Coral Health
and Disease: Developing a National Research Plan Coral Health
and Disease Consortium. Charleston, South Carolina, January
22-25, 2002.
Weil, E, Urreiztieta, I, Garzón-Ferreira, J. 2002.Geographic
variability in the incidence of coral and octocoral diseases
in the wider Caribbean. Proc 9th Int Coral Reef Symp, Bali.
In press.
Woodley, Cheryl. 2002. Memorandum. United
States Department of Commerce, National Oceanic and Atmospheric
Administration, National Ocean Service, Center for Coastal
Environmental Health and Biomolecular Research, Charleston,
South Carolina.
References to V. shiloi:
Banin, E., Israely, T., Fine, M., Loya,
Y., and Rosenberg, E. (2001a) Role of endosymbiotic zooxanthellae
and coral mucus in the adhesion of the coral-bleaching pathogen
Vibrio shiloi to its host. FEMS Microbiol. Lett.
199: 33–37.
Banin, E., Sanjay, K.H., Naider, F., Rosenberg, E. (2001b)
A proline-rich peptide from the coral pathogen Vibrio shiloi
that inhibits photosynthesis of zooxanthellae. Appl Environ
Microbiol 67: 1536–1541.
Banin, E., Ben-Haim, Y., Fine, M., Israely, T., and Rosenberg,
E. (2001) Virulence mechanisms of the coral bleaching pathogen
Vibrio shiloi. Proceeds of the 9th ICRS Symposium,
Bali (in press)
Banin, .E., Israely, T., Kushmaro, A., Loya, Y., Orr, E.,
and Rosenberg, E. (2000) Penetration of the coral-bleaching
bacterium Vibrio shiloi into Oculina patagonica.
Appl Environ Microbiol 66: 3031–3036.
Banin, E., Ben-Haim, Y., Israely, T., Loya, Y., and Rosenberg,
E. (2000) Effect of the environment on the bacterial belaching
of corals. Water, Air and Soil Pollut 123: 337-352.
Ben-Haim, Y., Banin, E., Kushmaro, A., Loya, Y., and Rosenberg,
E (1999) Inhibition of photosynthesis and bleaching of zooxanthellae
by the coral pathogen Vibrio shiloi.Environ Microbiol
1: 223–229.
Fine, M., Banin, E., Israely, T., Rosenberg, E., and Loya,
Y. (2001) Ultraviolet (UV) radiation prevents bacterial bleaching
of the Mediterranian coral Oculina patagonica. Mar Ecol
Prog Ser (in press)
Israely, T., Banin, E., and Rosenberg E (2001) Growth, differentiation
and death of Vibrio shiloi in coral tissue as a function
of seawater temperature. AquaticMicrobial Ecol 24:
1–8.
Kushmaro, A., Loya, Y., Fine, M., and Rosenberg, E. (1996)
Bacterial infection and coral bleaching. Nature 380:
396.
Kushmaro, A., Rosenberg, E., Fine, M., and Loya, Y. (1997)
Bleaching of the coral Oculina patagonica by Vibrio
AK-1. Mar Ecol Prog Ser 147: 159–165.
Kushmaro, A., Banin, E., Stackebrandt, E., and Rosenberg,
E. (2001) Vibrio shiloi sp. nov: the causative agent
of bleaching of the coral Oculina patagonica. Int
J Sys Evol Microbiol 51: 1383-1388.
Kushmaro, A., Rosenberg, E., Fine, M., Ben-Haim, Y., and Loya,
Y. (1998) Effect of temperature on bleaching of the coral
Oculina patagonica by Vibrio shiloi AK-1. Mar
Ecol Prog Ser 171: 131–137.
Rosenberg, E., Ben-Haim, Y., Toren, A., Banin, E., Kushmaro,
A., Fine, M., and Loya,Y. (1998) Effect of temperature on
bacterial bleaching of corals. In: Rosenberg E. (ed). Microbial
Ecology and Infectious Disease. Washington, DC: ASM Press,
pp 242-254.
Toren, A., Landau, L., Kushmaro, A., Loya, Y., and Rosenberg,
E. (1998) Effect of temperature on adhesion of Vibrio
strain AK-1 to Oculina patagonica and on coral bleaching.
Appl Environ Microbiol 64: 1379-1384.
Woodley, C.M., Downs, C.A., Fauth, J.E., Mueller, E., Halas,
J.C., Bemiss, J.A., Ben-Haim, Y., and Rosenberg, E. (2001).
A novel molecular diagnostic system to assess the physiological
status of corals. Proceeds of the 9th ICRS Symposium,
Bali (in press)
|
|
|