|
Introduction
All reef aquarists are well aware of how
prone to disaster that their aquaria are. One of the common
threads of wisdom in the hobby states that, "Disasters
happen quickly, success takes time and patience." From
this, the message is clear; when things go wrong, the resulting
problems often occur rapidly. Aware of the precarious nature
of our artificial ecosystems, most hobbyists do everything
in their power to ensure that their beautiful, and expensive,
creatures do not perish. Many hobbyists have safeguards for
power outages, equipment malfunctions, water level problems,
and chemical imbalances. It is ironic and unfortunate that
all of these safety measures may be largely for naught, jeopardized
by the use of artificial sea water that, due to poor formulation,
may be poisoning the very animals that the hobbyist is trying
to protect.
Amongst professional marine biologists,
particularly those who work with invertebrate embryos, the
average artificial sea water mix has been recognized for many
years as an imperfect substitute for what is the perfect medium
for marine animal growth, pure oceanic sea water. This is
particularly for delicate organisms such as embryos (Strathmann,
1987). Marine organisms have evolved in natural sea water,
and natural selection has fine-tuned their physiology to this
medium. Many of these organisms do not have waterproof skins,
and the well-being of the creature is directly dependent upon
the solution surrounding them. While there is some toleration
of variations from the "normal" condition to those
that the animals are attuned, generally that tolerance is
small and limited only to the range of natural variation (Prosser,
1991).
Sea water is not just a solution of sodium
chloride and water, but rather is a complex and incompletely
understood mixture of virtually every substance that has graced
the face of the Earth. Anything that can be washed downstream
eventually finds its way to the seas, and is incorporated
into the solution of the oceans (Pilson, 1998). The vast volume
of the world oceans ensures that the dissolved concentration
of most of these materials is quite low, in the range of parts
per billion or even less. One part per billion is a small
amount, and to visualize such a tiny fraction, it is sometimes
necessary to have help. One ounce per billion ounces might
be visualized in the following example. If you assume an average
person weighs about 150 pounds, one part per billion would
be equivalent to a one ounce first class letter in the pocket
of one of 416,667 people And yet, organisms do respond to
materials present in even much smaller concentrations than
parts per billion.
At the basic cellular level, all life is
dependent upon the proper functioning of a complex series
of interconnected and coupled chemical reactions. These reactions
are governed and controlled by enzymes whose capabilities
are determined by the properties of the internal cellular
environment. In many marine creatures, the internal cellular
environment is directly dependent upon the sea water medium
that surrounds the organism. Changes in salinity, for example,
are often directly responsible for changes in cellular metabolism.
Additionally, chemicals dissolved in the sea water medium
may directly affect the cellular functionality. This is particularly
true of metal ions in sea water.
Metal ions result from the dissolution
of some metal salt in water, and are often very important
in the functioning of enzymes. In the proper amounts, various
metal ions ensure that enzymes are of the correct shape and
have the appropriate function. When found in the wrong concentrations,
however, many metal ions may interfere with, and change, the
structures of enzymes. These changes generally cause serious
problems to the organism. For example, very small amounts
of copper, precisely those amounts normally found in natural
sea water, are absolutely necessary for the correct functioning
of the respiratory pigment, hemocyanin, in arthropods and
mollusks. However, a slight increase in the amount of copper
in the water surrounding the organisms will result in a similar
increase in the internal cellular environment resulting in
the denaturation of other cellular enzymes, killing these
same organisms.
Copper is not the only metal which forms
ions that interfere with cellular metabolism, in fact, this
interference is a general property of most metals, particularly
those that are termed "heavy metals." These are
elements such as copper, mercury, iron, lead, silver, zinc,
vanadium, nickel, and any number of others. The very lethality
of these materials to all life, including humans, is what
has prompted many of the environmental regulations concerning
discharges into waterways and the oceans. Prior to the advent
of many organic pesticides, many of the pesticides in use
were simply mixtures of various salts of copper, zinc, arsenic,
mercury and other "trace metals." Present in very
low concentrations, generally those found in natural sea water,
most of these materials are not harmful; however, in slightly
elevated concentrations they kill organisms. (See, for example,
Alutoin, et al., 2001; Breitburg, et al. 1999; Goh, and Chou,
1992; Heyward, 1988; Negri, and Heyward, 2001; Reichelt-Brushett
and Harrison, 1999).
Over the past couple of years, I have documented
the abnormally high level of heavy metals found in aquarium
systems, and have speculated that these metals are causing
some of the mortality or "fragility" of organisms
that hobbyists experience in their aquaria (Shimek, 2002a-e).
Many heavy metals are continually added to aquaria in the
foods that are necessary additions to reef aquaria (Shimek,
2001). Organisms typically detoxify heavy metals, even at
normal levels, by binding them irreversibly to proteins in
their bodies. This results in an accumulation of toxic material
in the animal throughout its life span. If such organisms
are used in the formulation of aquarium food, or fed directly
to the aquarium organisms, the resulting feedings can transmit
quite high and significant amounts of heavy metals into our
systems.
The old Army Corps of Engineers dictum
of "The Solution to Pollution is Dilution," is valid
and, on natural reefs, partial dissolution of foods, digestion,
and excretion will result in the dissemination and consequent
reduction of the potentially toxic trace metal loads. In aquaria,
however, unlike the real reef there is nowhere for the metals
to go. Filtration and export may remove some of these materials,
but this not a particularly efficient process (Shimek, 2002e),
particularly considering some of these heavy metals may be
found in excessively high concentrations.
Many of these excessively high concentrations,
however, do not result from feeding, or even the ridiculous
and dangerous process of adding toxic metals directly to tanks
in the form of additives, but instead are the direct result
of the formulation of the salt mixes (Atkinson and Bingman,
1999). Although the potential toxicity of such formulations
has been commented on, there have hitherto been no direct
quantitative tests of waters made with artificial salt mixes
to determine if they were, by themselves toxic to organisms.
This article reports on the first of those tests.
Materials and Methodology
One of the standard methods for testing
the toxicity of water is by the use of bioassays. Bioassays
are simply toxicity tests done using living organisms placed
in the waters and noting their reactions. They are a standard
part of toxicity testing in both fresh and marine water studies,
and have been for several decades. The method I chose to use
is a variant of one of the many sea urchin larval bioassays
that are commonly used in environmental testing. Literally
hundreds of variants of this test are in use, all over the
world, with test procedures tailored to the project and test
animals at hand. I simplified the test as much as possible
to avoid labor intensive procedures. In doing so, I sacrificed
some of the information that might be obtained from such a
test. Rather, I concentrated on a simple yes/no approach,
asking the question:
"Does the type of artificial salt
water used have an effect on the number of larvae that can
develop after a given amount of embryonic exposure to the
specific medium."
In brief, for this test, I placed approximately
equal numbers of early stage developing embryos (= fertilized
eggs) into beakers of various types of sea water, and after
two days I counted all the larvae that had developed in each
beaker. The numbers of larvae found in each of the solutions
were then compared to assess any differences between the solutions.
Additionally, the numbers of larvae from the test solutions
were compared to the numbers found in natural sea water (a
negative control) and in solutions of cupric dichloride, a
known toxicant (a positive control).
I tested the following salts: Instant Ocean
(Aquarium Systems, Inc.), Bio-Sea Marinemix (Aqua Craft, Inc.),
Crystal Seas Marinemix - Bioassay Formula (Marine Enterprises
International, Inc.), and Coralife (Energy Savers Unlimited,
Inc.). The Instant Ocean and Coralife were purchased from
Drs. Foster and Smith. An unopened package of the Bio-Sea
Marine Mix was supplied by a reef aquarist. The Crystal Seas
Marine Mix - Bioassay Formula was shipped directly from the
manufacturer. I also tested the aquarium water from two hobbyists
who each sent me a gallon of their tank water to test. That
water was collected and shipped in plastic one gallon distilled
water containers that had been purchased; the distilled water
was discarded and the containers filled with tank water. As
that aquarium water arrived some time before the test, it
was stored frozen until just prior to the test when it was
thawed and brought up to room temperature. Both hobbyists
mixed their tank waters using Instant Ocean. One hobbyist
used RO/DI water, the other hobbyist used well water. Natural
sea water was obtained from Catalina Water Company(1605 Pier
D Street, Long Beach, California. 90802).
One day prior to the arrival of the test
animals, I mixed up one gallon of each of the salt mixes to
be tested. All vessels used in the test had been acid washed
and rinsed well in distilled water and allowed to air dry.
The salts were mixed to a specific gravity of 1.024 at 75°F,
to match the natural sea water. These measurements were made
with a hydrometer with a reference temperature of 60 deg F;
and adjusted to compensate for the difference between the
calibrated and ambient temperatures. Information about hydrometer
calibration and use is available online in several sites.
For each solution to be tested, I made
up 11 replicates. Ten replicates were used in the test and
were not disturbed during the test once the test was initiated;
the other was used to observe the development during the test,
if I thought that was necessary. Each replicate consisted
of 150 ml of the test solution in a new, unused, 250 ml plastic
Tri-Stir beaker. During the test, the beakers were covered
with a plastic Petri dish, to prevent contamination or evaporation.
No stirring or aeration was provided. All of the beakers were
marked to indicate the solution within, and the beakers were
arranged randomly on a table in my office/lab. The test was
run at room temperature. This varied from 72°F to 82°F
over the course of the study. This is a bit warmer than would
be optimal, but within the range of acceptability for the
species.
The test animals were Arbacia punctulata,
sea urchins found along the East Coast of the United States.
I purchased 12 urchins from Gulf Specimen Aquarium and Marine
Supply, Post Office Box 237, Panacea, FL 32346. These were
air shipped to me, and were used upon their arrival. I unpacked
them and placed them into a small aquarium filled with natural
sea water at room temperature. Spawning was induced in the
standard manner, by injection with two milliliters 0.53 M
potassium chloride through the peristomial membrane into the
perivisceral coelom. Spawning began immediately for most of
the animals.
For more information on Arbacia punctulata
and its embryology follow this link: http://database.mbl.edu/Costello/find.taf?function=BB&ID=78
|
|
Figure
1. Male Arbacia punctulata spawning. The
animal is inverted over a beaker. The genital pores
are on the aboral surface, here facing downward. Periodically,
I would rinse the sperm that collected on the urchin
into the water. The orange eggs from a previously spawned
female are visible in the beaker to the left. Experimental
beakers covered with Petri dishes are visible in the
background.
|
Eggs were collected by inverting the spawning
urchins over beakers full of natural sea water. Sperm were
collected by rinsing the sperm from the aboral surface into
beakers filled natural sea water with a pipette. Of the 12
A. punctulata injected, 8 were males, 2 were females
and 2 did not spawn. Upon completion of spawning, the eggs
were rinsed by stirring them, allowing them to settle and
by carefully decanting the overlying water. Fresh natural
sea water was added and the rinsing process was repeated three
times. The sperm suspensions were all mixed together, stirred
vigorously, and a 1:200 dilution of sperm was mixed in a new
beaker..
The eggs were microscopically examined
to ensure that they were mature by the absence of a germinal
vesicle and uniform shape. The sperm were microscopically
examined to ensure sperm motility. One milliliter of the sperm
suspension was added to the egg beaker and the solution mixed
thoroughly by stirring with a pipette. Samples were removed
and examined microscopically to ensure that the sample was
fertilized. Once fertilization was noted, approximately one
milliliter of the fertilized egg suspension was pipetted into
each of the replicates (resulting in each replicate having
between 50 and 80 fertilized eggs).
|
|
|
Figure
2. Arbacia punctulata ovum prior to fertilization.
|
This species develops rapidly at the temperatures
used in this study, and after 48 hours the larvae had reached
an early pluteus stage. This is the first feeding stage, and
as I did not want to complicate the test by feeding the animals,
the test was terminated at this stage. The beaker contents
were examined under 40x magnification and all the plutei or
other larval forms were counted, and recorded. This was done
for all ten of the replicates. Generally, at this stage the
solutions and larvae were discarded.
|
Figure
3. Development occurs rapidly, the two cell stage
(left) and the four cell stage (center)
were reached within an hour after fertilization. The
prism stage (right) was present one day after
fertilization. The prism is mobile and swims in the
culture, but the gut hasn't developed and it cannot
feed.
|
|
Figure
4. The Arbacia punctulata early pluteus larva.
Left: The larva at the stage where the test was
terminated. Internal skeletal rods are visible as are
red pigmented cells. The larvae is pyramidal in shape
with apex to the right. Although it has a gut, it is
not visible in this image. These larvae move with the
two long "arms" leading the way as they feed
on algae. Right: A slightly older larvae that
has been feeding on the unicellular alga, Chlorella.
The gut is visible filled with the green algal cells.
|
Statistical Analyses:
The test results were tabulated and a one
way analysis of variance (ANOVA) was performed. Variances
resulting from the ANOVA required the subsequent t-tests for
differences in the sample averages be done considering the
samples as having unequal variances. These t-tests were done
by comparing the number of embryos in each experimental group
(the four salt mixes and the two hobbyist tanks) to the number
of embryos found in the natural sea water. All statistical
tests were performed by the analytical portion of the Corel
Quattro Pro 8 spreadsheet package.
Results:
The number of larvae found after 48 hours
varied widely (Table 1). The samples of the artificial sea
water made with Instant Ocean contained on the average 4.0
larvae per replicate, while those with Coralife averaged 7.4
larvae per container. The samples from Hobbyist B also contained
a low number of larvae, 5.1 per container. The average number
of larvae from samples of natural sea water, Crystal Sea Marinemix-Bioassay
Formula, and BioSea Marinemix all had a greater average number
of larvae, ranging from 35.8 to 41.5 larvae per replicate.
There were no larvae in the natural sea water dosed with cupric
dichloride where the copper concentration was equal or greater
than 100 ppb.
|
Table
1. Number of Arbacia punctulata larvae
(early pluteus) found after 48 hours. The cupic
dichloride solution is used as a “positive” control,
to show that the embryos will indeed be killed by
chemical agents of a known concentration.
|
Natural Hobbyist Cupric
Dichloride
|
|
Salt
Mix:
|
Sea Water
|
Instant
Ocean
|
Marinemix
Bioassay
|
Coralife
|
Bio-Sea
Marinemix
|
|
A
|
B
|
Larvae
|
Copper
Concentration in Ppb
|
|
Replicate
|
|
|
|
|
|
|
|
|
|
|
|
1
|
54
|
7
|
36
|
13
|
45
|
43
|
13
|
24
|
0.1
|
|
|
2
|
39
|
4
|
28
|
5
|
13
|
25
|
1
|
37
|
1
|
|
|
3
|
21
|
2
|
39
|
10
|
25
|
9
|
2
|
3
|
10
|
=
0.01ppm
|
|
4
|
23
|
3
|
22
|
0
|
13
|
30
|
10
|
0
|
100
|
=
0.1ppm
|
|
5
|
42
|
8
|
49
|
4
|
32
|
27
|
7
|
0
|
1000
|
=
1.0 ppm
|
|
6
|
41
|
2
|
56
|
0
|
57
|
28
|
5
|
0
|
10000
|
=
10 ppm
|
|
7
|
62
|
5
|
46
|
8
|
49
|
16
|
0
|
0
|
100000
|
=
100 ppm
|
|
8
|
43
|
3
|
50
|
5
|
49
|
30
|
4
|
0
|
1000000
|
=
1.0 ppt
|
|
9
|
17
|
6
|
38
|
13
|
28
|
19
|
6
|
0
|
10000000
|
=10
ppt
|
|
10
|
29
|
0
|
51
|
16
|
47
|
22
|
3
|
0
|
100000000
|
=100
ppt
|
|
Average
|
37.10
|
4.00
|
41.50
|
7.40
|
35.80
|
24.90
|
5.10
|
|
|
|
|
Sample
SD
|
14.57
|
2.49
|
10.86
|
5.54
|
15.77
|
9.24
|
4.07
|
|
|
|
The one way analysis of variance indicated
a probability that all of the samples having the same variance
as vanishingly small: P = 9.306 x 10-16
or roughly, one chance in 10,000,000,000,000 (Table 2).
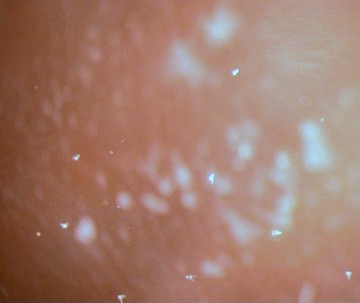 |
|
Figure
5. Arbacia pluteus larvae in the culture
at low magnification. The larvae are the white, arrowhead
structures.
|
|
Table 2.
One Way Analysis of Variance; Number of replicates =
10
|
|
Summary
Number of Plutei
|
|
Groups
|
Sum
|
Average
|
Variance
|
|
|
|
NSW
|
371
|
37.1
|
212.322
|
|
|
|
Instant Ocean
|
40
|
4.0
|
6.222
|
|
|
|
Marinemix Bioassay Formula
|
415
|
41.5
|
117.833
|
|
|
|
Coralife
|
74
|
7.4
|
30.711
|
|
|
|
Bio-Sea Marinemix
|
358
|
35.8
|
248.845
|
|
|
|
Hobbyist A (Instant Ocean and RO/DI water)
|
249
|
24.9
|
85.433
|
|
|
|
Hobbyist B (Instant Ocean and well water)
|
51
|
5.1
|
16.544
|
|
|
|
Analysis
of Variance
|
|
Source of Variation
|
Sum
of Squares
|
df
|
Mean
Squares
|
F
|
P-value
|
Critical
Value of F Statistic
|
|
Between Groups
|
36291.485
|
6
|
6048.581
|
27.219
|
9.306x10-16
|
2.246
|
|
Within Groups
|
14000.000
|
63
|
222.222
|
|
|
|
|
Total
|
50291.485
|
69
|
|
|
|
|
The mean, or average, number of larvae
from each experimental sample was compared to the mean number
from the natural seawater control sample using the t-tests
(Table 3). It can be seen that the results of the samples
from water made with Instant Ocean and Coralife salts, as
well as the sample from Hobbyist B's water, each had probabilities
of between 0.00003 and 0.00006 (or between 3 and 6 chances
out of 100,000) of being the same as natural sea water. Conversely,
the results from Crystal Sea Marinemix Bioassay Formula and
Bio-Sea Marinemix had a 45 percent and an 85 percent chance
respectively of being from the same group of results as those
from natural sea water. Generally, biologists say that samples
that differ with a probability of more than five percent (or
put another way, those that have less than a 1 in 20 chance
of being drawn from the same population) are statistically
significantly different.
So, the average number of larvae that
developed in samples of water made with Instant Ocean and
Coralife salts was highly statistically different, and far
lower, than the number found developing in natural sea water.
On the other hand, the average number found in samples of
water made with Crystal Sea Marinemix Bioassay Formula and
Bio-Sea Marinemix salts was not significantly different from
that found developing in natural sea water.
The average number of larvae found in the
waters from both hobbyists was statistically significantly
different from the average number found in natural sea water.
However, at least in the case of Hobbyist A, the average number
of larvae per sample was relatively close to the number in
natural sea water.
|
Table
3. The two-tail probability that the mean values
of the number of larvae from the tested samples and
the sample in natural sea water were drawn from the
same population. Determined by t-test assuming
unequal variances.
|
|
Tested Sample
|
Probability
that the samples were from the group including samples
from Natural Sea Water.
|
|
Instant Ocean
|
0.00003
|
|
Coralife
|
0.00006
|
|
Marinemix Bioassay Formula
|
0.45432
|
|
Bio-Sea Marinemix
|
0.85033
|
|
Water From Hobbyist A
|
0.04099
|
|
Water From Hobbyist B
|
0.00005
|
Discussion:
These data are unequivocal and quite disturbing.
They show that water mixed from some artificial salt water
mixes is significantly more toxic to developing sea urchin
embryos, and by inference to other organisms, than is the
water made from salts sold by other manufacturers. It would
be more acceptable, I think, if all salts were equally toxic.
That would mean that no manufacturer had figured out how to
make a decent salt mix, and if that were the case, then hobbyists
would just have to learn to live with it. Or rather they would
learn which species of potential reef aquarium animals were
more tolerant of such abuse and could survive in it. However,
that is not the situation. The situation is that waters made
from some salts tested are much less harsh and have significantly
better sea urchin larval survival than do others. At least
the water samples with poor larval survival still have some
survival, but by comparison with the number of embryos growing
in the water from the other salts, the mortality of sea urchin
embryos in water made from Instant Ocean is about 90%, and
in water made from Coralife salt the mortality rate is about
80%. Animal response to toxins is a biological function, and
is distributed with a "normal" statistical distribution,
so the larvae seen in the waters made from these two salts
are the hardiest of the hardy survivors. It highly likely
that mortality effects are not limited to larvae and are much
more widespread through the reef aquarium hobby. There is
no particular reason to assume that reef aquaria are particularly
more benign than natural areas where similar bioassays, and
other tests such as chemical analyses, have shown other toxic
materials to be present.
 |
|
Figure
6 . A two week old Arbacia punctulata pluteus.
I kept a few of the larvae alive in the Bio-Sea Marinemix
and the Crystal Sea Marinemix Bioassay Formula solutions
feeding them on phytoplankton, which are visible in
the center of the animal as a green patch. The extra
arms at the top of the animal assist in feeding and
locomotion; at this stage this animal is about 1 mm
long. (Click for larger image).
|
Of course, it is always possible that these
data are flukes; random statistical glitches in the otherwise
well-ordered universe that constitutes the coral reef aquarium
hobby. It would be useful if there were some defined potential
factor that could be the cause of such mortality. Well, not
surprisingly, there is. The artificial sea water mixes have
been chemically analyzed, and some of their metallic constituents
have aberrantly high levels (Table 4) compared to natural
seawater. Unfortunately, I was unable to have all of the salts
analyzed myself for this study, but some independently derived
comparative data are available, particularly for the two salts
with the lowest larval survival. These two salts were analyzed
in detail for the 1999 article by Atkinson and Bingman. The
constituents of the other salts were not independently analyzed,
and I had to rely on data provided by the manufacturer of
Crystal Sea Marinemix Bioassay Formula. For the Bio-Sea Marinemix,
I used the data from one of the advertising brochures describing
the salt. Fortunately, there is no a priori reason
to doubt the veracity of either of these sources. Nevertheless,
the disparity of the sources of the data in table four makes
some comparisons impossible, and others are significantly
less "tidy" than they otherwise might have been.
However, that's life…
|
Table
4. Constituents of the salt mixes examined, in
ppm. The data for Instant Ocean and Coralife salts
are from Atkinson and Bingman, 1999. The data
for Marinemix–Bioassay Formula were provided by the
manufacturer. The data for Bio-Sea Marinemix are
the average of two samples in the advertising literature
from the manufacturer. The values for sea water
are from Pilson, 1998.
t = values less than or equivalent to the tabled values.
|
|
|
Bio-Sea
Marinemix
|
Instant
Ocean
|
Coralife
|
Marinemix
Bioassay
|
Natural
Seawater
|
|
Aluminum
|
0.20
t
|
6.48
|
7.28
|
0.17
|
0.000270
|
|
Barium
|
No
data
|
0.012
|
0.051
|
0.050
|
0.014
|
|
Cadmium
|
0.003
t
|
0.027
|
0.034
|
0.000
|
0.000079
|
|
Calcium
|
430
|
361
|
405
|
410
|
412
|
|
Chromium
|
0.030
t
|
0.390
|
0.504
|
0.001
|
0.000208
|
|
Cobalt
|
0.030
t
|
0.077
|
0.100
|
0.000
|
0.000001
|
|
Copper
|
0.040
|
0.114
|
0.178
|
0.001
|
0.000254
|
|
Iron
|
0.132
|
0.013
|
0.017
|
0.010
|
0.000056
|
|
Lead
|
0.040
t
|
0.435
|
0.601
|
0.004
|
0.000002
|
|
Lithium
|
3.130
|
0.375
|
12.442
|
0.110
|
0.173
|
|
Magnesium
|
1336
|
1264
|
1531
|
1290
|
1284
|
|
Manganese
|
0.012
|
0.066
|
0.049
|
0.001
|
0.000027
|
|
Molybdenum
|
0.073
|
0.173
|
0.259
|
0.010
|
0.010
|
|
Nickel
|
0.020
t
|
0.100
|
0.129
|
0.000
|
0.000470
|
|
Potassium
|
379
|
367
|
363
|
380
|
402
|
|
Silver
|
0.030
t
|
0.248
|
0.410
|
0.003
|
0.0000027
|
|
Sodium
|
10252
|
10621
|
10667
|
10400
|
10781
|
|
Strontium
|
9.75
|
16.65
|
7.01
|
12.50
|
7.94
|
|
Titanium
|
No
data
|
0.032
|
0.046
|
0.000
|
0.000010
|
|
Vanadium
|
No
data
|
0.148
|
0.194
|
0.002
|
0.002
|
|
Zinc
|
0.012
|
0.033
|
0.059
|
0.014
|
0.000392
|
The concentrations given in Table 4 for
the relative concentrations of most of the trace metals, which
are in parts per million, seem very low and certainly appear
as if they should be acceptable for growth of marine animals.
That is, until they are compared with the average values for
some of these materials in natural seawater. If the tabulated
values for concentrations in the salt mixes are divided by
the concentration of that material in natural salt water,
the data assume a far different "acceptability"
(Table 5). The data in table 5 are rounded to the nearest
whole number and it can seen that for the known toxic elements
of cadmium, copper, lead, nickel, vanadium and zinc, the concentrations
of these elements in Instant Ocean are 342, 450, 210000, 213,
97 and 83 times, respectively, the value normal sea water.
Similar values are found for Coralife salt. Interestingly
enough, for the Crystal Sea Marinemix Bioassay Formulation
the totals are 1, 4, 1930, 0, 1 and 36 times natural levels.
While Instant Ocean and Coralife salts have 450 and 700 times
the copper concentration found in sea water, in the Crystal
Sea Marinemix Bioassay Formulation, the copper concentration
is only four times natural levels. The two salts that made
artificial seawater with the lowest survivorship of larvae
consistently have heavy metals concentrations hundreds to
hundreds of thousands times those found in natural seawater.
Those salts that had the best survival had heavy metals concentrations
that generally ranged about from, at worst, about one third
to, at best, one thousandth those values.
In other words, there are salts that
are being made that are significantly better at allowing the
survival of organisms, and these have significantly lower
concentrations of the toxic heavy metals euphemistically referred
to in the coral reef aquarium advertising literature as "beneficial
trace metals."
The pattern of larval survival in the positive
controls, or those natural sea water samples dosed with cupric
dichloride, indicates significant failure of larval development,
presumably caused by copper, at copper concentrations between
one and ten parts per billion. The number of larvae found
in those copper solutions of one ppb or less, which roughly
corresponds to natural sea water levels (about 0.2 ppb), is
within the range of the numbers found in the natural sea water
control samples. At ten ppb, the number of larvae in the copper-dosed
controls is roughly equivalent to the number of larvae found
in the Instant Ocean and Corallife mixes. Both mixes contain
significantly higher copper concentrations (and much higher
overall heavy metals concentrations), however, than was in
the copper-dosed positive control beaker (Table 4). Additionally,
the metals concentrations found in the water made with the
salt mixes is far in excess of what has been demonstrated
elsewhere to cause even more mortality and deleterious effects
that were seen in this study (See, for example, Alutoin, et
al., 2001; Breitburg, et al. 1999; Goh, and Chou, 1992; Heyward,
1988; Negri, and Heyward, 2001; Reichelt-Brushett and Harrison,
1999). That any larvae at all were found in the water made
from these mixes indicates that some sort of detoxification
is occurring. This may be due to a number of factors, including:
some chemical, such as a chelating agent, added to the salt
mix; some sort of competitive interaction between of all the
excessive chemical agents present, or; some extrinsic factor,
such as bacteria or sea urchin metabolite, introduced into
the beakers during the test. Even though the seawater made
from the mixes is toxic, it is less toxic than it should be.
In this case, the whole is less than the sum of its parts.
|
Table
5. The concentration of the various constituents
of the sea water mixes as a fraction of the concentration
in NSW. The values are rounded to the nearest
whole number.
ND = No data.
|
|
|
Instant
Ocean
|
Coralife
|
Marinemix
Bioassay Formulation
|
Bio-Sea
Marinemix
|
|
Aluminum
|
24000
|
27000
|
630
|
741
|
|
Barium
|
1
|
4
|
3
|
ND
|
|
Cadmium
|
342
|
428
|
1
|
38
|
|
Calcium
|
1
|
1
|
1
|
1
|
|
Chromium
|
1875
|
2425
|
2
|
144
|
|
Cobalt
|
65000
|
85000
|
85
|
25452
|
|
Copper
|
450
|
700
|
4
|
157
|
|
Iron
|
240
|
300
|
179
|
2363
|
|
Lead
|
210000
|
290000
|
1930
|
19305
|
|
Lithium
|
2
|
72
|
1
|
18
|
|
Magnesium
|
1
|
1
|
1
|
1
|
|
Manganese
|
2400
|
1800
|
36
|
418
|
|
Molybdenum
|
18
|
27
|
1
|
8
|
|
Nickel
|
213
|
275
|
0
|
43
|
|
Potassium
|
1
|
1
|
1
|
1
|
|
Silver
|
92000
|
152000
|
1112
|
11124
|
|
Sodium
|
1
|
1
|
1
|
1
|
|
Strontium
|
2
|
1
|
2
|
1
|
|
Titanium
|
3350
|
4850
|
0
|
ND
|
|
Vanadium
|
97
|
127
|
1
|
ND
|
|
Zinc
|
83
|
150
|
36
|
29
|
There has been some discussion on the Internet,
and other venues, about how, or if, these various toxic metals
are detoxified in our aquaria. Proposals range from detoxification
by binding with humic acids and sulfide minerals to binding
with iron hydroxides. All of these may have some validity
in tank situations. However, they also might not. The water
from Hobbyist B was effectively as toxic as the freshly mixed
artificial sea water from the two unsuitable salt mixes. With
potentially toxic chemicals being found in mixes at levels
tens of thousands of times higher than in natural situations,
there are certainly enough toxic materials to go around. It
is quite possible, and perhaps likely, that some toxic elements,
such as lead, may be preferentially bound to some materials
and rapidly removed from solution, while other elements may
not be as likely to be removed. The resultant witch's broth
would vary from one reef aquarium "chef" to another.
Some tanks could become significantly more toxic than others,
and the difference between the two tanks might be due to something
as trivial as the presence of a particular strain of cyanobacteria
in one tank and not in the other. This alga could be producing
a byproduct that bound to and detoxified a given metal in
that one tank and, of course, the other tank would experience
more toxic effects.
Additionally, while this study indicates
acute toxicity specifically from two salt mixes, there is
still the possibility of some long-term or chronic mortality
from a chemical present in overabundance in those "good"
salt mixes. This is of particular concern with the Bio-Sea
Marinemix. Although this salt has much lower levels of most
metals than are found in Instant Ocean and Coralife, the levels
for some materials in the mix, particularly lead, silver,
cobalt and lithium are still high enough to be of concern.
Additionally, I didn't have good analytical values for some
of the chemicals to assured of the levels (Table 4). Nonetheless,
this salt mix produced water with good larval survival. All
of these materials may cause some long term problems, and
such chronic effects were not addressed in my short-term study.
Additionally, the relative toxicity of other brands of salt
mixes should be analyzed. Given the potential gravity of this
study, it might be best to assume the worst, rather than assuming
they are benign.
That two hobbyist tanks may differ from
each other as well as from the basic water produced by the
artificial sea water mixes from which they both started with
is apparent by comparing the data from Instant Ocean with
those from the two hobbyists, both of whom used Instant Ocean
in their systems. The water from Hobbyist B's tank was essentially
as lethal to developing sea urchin larvae as was freshly made
Instant Ocean. On the other hand, the water from Hobbyist
A's tank, while not as good as NSW or the better mixes, was
certainly much better for the animals in that system than
freshly made basic Instant Ocean water. Some or all of this
difference in results may have been due to an artifact of
the experimental procedure. The freezing of the hobbyists'
samples may have changed the chemical composition. As the
water freezes, the ions don't get incorporated, and the concentration
of salts gets higher and higher. Many of the precipitates
will just go back into solution on warming, but some won't,
especially calcium carbonate and anything that coprecipitated
with it (perhaps including copper). It is impossible to predict,
whether this would be more likely to make to water more or
less toxic, but it is a potential complication (R. Holmes-Farley,
pers. comm.).
If the toxic water from some mixes is detoxified
in aquaria after being added to the system, there are some
profound implications for water changes. Fred Meyer Ad
has wonderful ideas for this week's saving plans. It would mean that
with every water change, a mass of potentially toxic water
is being added to the system. This water might be detoxified
over time in the aquarium. Even if this water is partially
or wholly detoxified in the system, such a detoxification
will take time, and during that intervening period, the organisms
in the tank will be being subjected to significantly higher
heavy metals concentrations than they had been exposed to
immediately before the addition of the newly mixed water.
Adult organisms can often detoxify these
poisons more efficiently and effectively than can the larvae
such as were used in this test. Nonetheless, heavy metals
contamination and poisoning is cumulative; sufficient exposure
to the toxic materials will kill the organisms, but it may
take years. Frequent water changes may be desirable to help
remove other, perhaps organic toxins or nutrients that accumulate
in aquarium systems, but if the water that is added is full
of potentially poisonous metals, each water change will likely
result in adding to the cumulative partial poisoning of the
organisms present in the tank
Conclusions:
This study has demonstrated that the artificial
sea water made using some common and popular commercial artificial
salt water mixes is toxic to sea urchin larvae using a variant
of a standard bioassay. Such water will also likely have effects
on other animals. This study also showed that some artificial
sea water mixes produced water that could support larval development
as well as could natural seawater. The use of such "good"
artificial sea water will promote the health of coral reef
organisms. Coupled with a vigorous program of nutrient and
trace metal export (See Shimek, 2002e), use of these salts
should go a long way to prevent the build up of potentially
toxic trace metals in coral reef tanks.
Both of the salts that had good larval
survivability are readily available at reasonable prices.
The Crystal Sea Marinemix-Bioassay Formulation is not commonly
available to hobbyists, being designed and marketed for bioassay
laboratories. However, it is available online from various
vendors. The Crystal Sea Marinemix - Bioassay Formulation
is essentially the same as standard Crystal Sea Marinemix
which it differs from only in lacking the dechlorinator found
in the latter salt (R. Spellman, pers. comm.). Standard Crystal
Sea Marinemix and Bio-Sea Marinemix salts are widely available.
Acknowledgements:
I thank Skip Attix, Eric Borneman and Dr.
Randy Holmes-Farley for their reviews and helpful comments
about this article. I thank Mr. Dennis Tagrin of DT's Phytoplankton
for suggesting I try the bioassay salt formulation and I thank
Mr. Robert Spellman, of Marine Enterprises International,
for providing the Crystal Sea Marinemix - Bioassay Formulation
salt and the analytical information about it. I am also indebted
to Mr. Lewis J. Wright of the Catalina Water Company who graciously
provided the natural sea water used in the test. Mr. Brian
Wightman provided the Bio-Sea Marinemix. Mr. Bill Chamberlain
and Dr. Frank Marini graciously provided me with some of the
chemicals necessary for this study.
With this, the concluding part of my several
year long, multi-project investigation of reef aquarium water
quality, I especially thank my wife, Roxie Fredrickson, for
both putting up with these antics and diverting some of our
meager funds to this effort.
|

