|
Over the last several years, so-called
"sand beds," essentially layers of sand of various
thicknesses and arrangements, have come to be common fixtures
in marine reef aquaria. The use of these sand beds has been
correlated with significantly increased survivability of many
organisms in reef aquaria, particularly when compared to the
bare bottom tank arrangements that were in the vogue about
a decade ago. Nevertheless, few hobbyists seem to realize
why sand beds should contribute to the success of their tanks,
and fewer yet seem to understand how those beds work.
Over the last five years, I have made much
of the point that our aquaria are artificial ecosystems, or
microcosms, representative of the real reef environment. Of
this there can be no doubt; our systems mimic relatively well
many of the processes occurring in the natural world and,
when populated by an appropriate group of organisms, many
of the interactions occurring in natural habitats occur in
aquarium systems. The approach of dealing with aquaria as
artificial ecosystems has been criticized primarily on the
basis that reef aquaria are patently artificial. However,
such criticisms are both rather silly and quite wrong. Reef
aquarium systems have to be quite good approximations of the
real world, otherwise those animals that are kept in them
would not be doing as well as they are. The organisms don't
know they are not in the natural environment and any coral
reef organism has evolved to deal with an environment that
has as its limits those same limits as are found on real reefs.
The fact that we can deal with aquaria
as good mimics of the real thing allows us to use "real
world" or "scientific" data both to troubleshoot
problems and to advance the techniques of animal husbandry.
As we know that organisms must live within the ranges of their
tolerances, we can recognize that problems will occur when
something in an aquarium is far outside that range, such as
the excessively high concentrations of poisonous heavy metals
found in some artificial sea water mixes . Once that recognition
is made, we can adjust for the problem and proceed. In this
way, we may incrementally increase our understanding of the
animals and what we need to do to maintain them.
If aquaria are artificial ecosystems, however,
the component that is least artificial is the sand bed. This
part of a reef aquarium, with little input from the aquarist,
functions much as do the sandy areas near a real reef. That
functionality is due to a rather complex interaction of physical
and biological factors, but most of those interactions are
unseen, and, I think, unappreciated by the average aquarist.
Without those interactions our reef aquaria would simply fail.
The fact that they don't fail is a tribute to the ease of
constructing this one major functional analogue to an extremely
critical coral reef community.
Sediments In Reefs
Sand beds are constructed physically of
sands, and sand is defined as unconsolidated sediments made
of particles between one sixteenth of a millimeter and two
millimeters in diameter. Coarser sediments are referred to
as gravel, finer ones as silts and clays (Holme and McIntyre,
1984). Of course, in the real world there is a continuum of
sizes found in these environments, and the sediments actually
found in any one spot reflect not only the geological and
biologic history of the area, but also the hydrographic regime
of the area. In other words, what is present is the result
of what is available that hasn't been washed away by the waves.
Figure
1. A figure from Holme & McIntyre, 1984, showing possible
ways of representing the variation among sediment types. All
of these types of sediments will likely be found in various
coral reef environments.
Sands surrounding natural coral reefs may
be made of a number of substances. Around volcanic islands
there are often regions of volcanic lava sand. Volcanic islands
are the basis for most coral atolls and many fringing reefs,
so lava sands are commonly found around reefs in nature. Coral
reefs located near areas of river mouths or extensive areas
of runoff often are surrounded by silica sands or fine sediments
of other upland or inland sources. Of course, coral reefs
may be surrounded by calcareous sands resulting from the breakdown
of corals and other calcifying organisms. Calcareous sands
may also be formed by the precipitation of particulate calcium
carbonate in coral lagoons, one of the natural sources of
oolitic sand. Calcareous sands may also be formed from the
skeletal breakdown of other organisms, such as foraminiferans,
bivalves, calcifying algae, or barnacles.
The chemical composition of the sands has
a small effect on the organisms found in the sands, but that
effect is minor compared to the effects due to differences
in sediment particle size distribution. The sediment particles
found in any given area are primarily due to the effects of
sediment movements caused by wave action and water currents.
Sediment density has some effect on what is present, but basically
for any given sediment, finer particles will be found in areas
with less water movement. Consequently, the pattern of sediments
surrounding a coral islet that is one part of a coral atoll
will be a complex tapestry of sediment sizes. In general,
coarser sediments will be found in areas of higher current
flow and wave action while finer sediments will predominate
in areas of less kinetic energy. The absolute position of
the sediments will often vary from season to season, particularly
in intertidal and shallow subtidal areas. Tourists who always
visit a given resort at a particular time of year are often
quite amazed when they return to the resort six months out
of sync with their usual pattern and find the sandy beach
they expect to see has vanished, leaving a hard coral pavement
instead. Movement of sediments is less in deeper waters but
it still occurs. In fact, one major characteristic of natural
sand beds is their mobility.
Sediments in any one spot may be characterized
by several discrete parameters. The first parameter is the
average, or mean, sediment particle size. The second factor
is the shape of the cumulative sediment particle size distribution;
if a sample of the sediment is taken and the diameters of
the sediment particles measured, the resulting graph will
be a bell or "normal" curve centered around the
average particle size. How that bell curve deviates from an
ideal statistical bell curve reveals a lot about the sediments.
For example, at the extremes, the curve may be low, broad,
and flat or quite narrow and high. In the former case, it
indicates a wide variety of sediment particles in each sample
which, in turn, indicates lesser effects due to waves or currents.
In the latter case, the sediments will be almost all of the
same size, indicating a lot of movement of sediment particles
by wave action and the resulting "sorting" of them
by size. Well-sorted sediments are quite characteristic of
areas with high currents or strong wave action, while poorly
sorted sediments with a wide variety of particle sizes are
characteristic of calmer waters. The third important parameter
is the amount of organic material found in the sediments.
I have worked in areas where the organic content was effectively
zero. At the other extreme, I have sampled some areas where
the organic content of non-polluted sediments was as high
as about twenty percent by weight. In polluted areas the organic
content may be even higher.
The size distribution of sediments in any
marine soft sediment area is critical to the determination
of the organisms living in those sediments. Organisms live
on, and between sand grains, and the mixture of the sizes
of the grains is critical. Sand grains of inappropriate sizes
may be too big to move or, conversely, too small to be stable.
Additionally, the mixture of the various sizes determines
the ease with which water moves through the sediments.
Here are a couple of links showing bacteria
on individual sediments:
Ocean
Explorer
http://www.rnw.nl/science/assets/images/020903bacteria.jpg
There is obviously a complex interplay
of factors then that determines the natural sediments found
around a coral reef. Organic materials may come from the reef,
adjacent areas, or from inland runoff. Tidal, wave, and storm
patterns all influence the kinetic energy that will be transmitted
to the sediments. Geological factors such as the presence
of submerging or emerging coastlines may also contribute to
the types of sediments present.
 |
Figure
2. Image taken through the viewing port of a research
submersible. The depth was 165 feet. Note the ripple
marks and the relatively large sediment particle sizes
(the ripples are about 6 feet away from the port and
are over 2 feet high). This area receives occasional,
but regular, winter storm waves in excess of 60 feet
high. Shallow water habitats in this area have no sand
whatsoever; the smallest particles are about 6 feet
in diameter. Water action is the ultimate determinant
of sediment particle size in natural environments.
|
|
Figure
3. Image taken from the inside of the Plexiglas
sphere of the research submersible, Johnson Sea Link
II. The flat featureless sandy substrate visible in
search light glow is about 8 feet away (or about as
far as the sand and gravel seen in the preceding figure).
This picture was taken off the outer edge of the coral
reef of the Bahamas in 1140 feet of water. Here there
is mild gentle current, and the sediment is a well-sorted
fine white sand.
|
 |
In aquaria, the sediments are chosen by
the aquarist and added to the system; however, that is only
the beginning of the development of the sediments. As in the
natural world, aquarium sediments are dynamic, albeit on quite
a different scale than is seen in nature. Both the sediment
particle size and physical distribution and amount of organic
material in aquarium sediments will change through time.
The change in aquarium sediment particle
distribution is most evident in fine calcareous sediments.
The average particle size will tend to decrease in these sediments
as particles are eroded or dissolved. In aquaria with poor
carbonate buffering, there will be a tendency for finer particles
on the surface to partially or wholly dissolve. Additionally,
when deposit-feeding animals eat the sediments to digest the
bacteria and algae off of them, some fraction of the sediment
particles is also likely dissolved. Organic material will
be added to the sediments and some of this will get incorporated
into the sediments as fine particulate material. Often, this
fine organic material is the site of organo-metal complexes
forming an insoluble precipitate of toxic materials. Such
precipitates are typically very small. The net result of all
of these processes is that the average size of the sediment
particles decreases in size over time.
Sediments and Water
In either the real reef or in aquaria there
are some aspects of the sediments and water that are important.
First, it is important to realize that passive water movement
through the sediments is essentially impossible. The channels
between the sand grains are so small that the resistance to
passive water movement is, for all practical purposes, absolute.
Unless the water is pumped through the sediments, it simply
doesn't move. Contrary to a lot of reef aquarium mythology,
water does not "diffuse" through the sediments.
Materials dissolved in the water may diffuse within the water
medium, but that movement is very slow and generally inconsequential.
As we will see, unless the aquarist arranges for some sort
of active pumping, all water movement in aquarium sediments
is mediated by organisms.
Water flow over the sediments may be either
turbulent, such as caused by a power head, or laminar, such
as uniform bulk water flow. Turbulent flow will move some
water through the upper few fractions of an inch of sediments,
laminar flow generally will not. However, in either case,
there will be little real interchange of water from the sediment
interstices into the water column and vice versa. Even in
aquaria with strong surge devices, as long as the sediment
is not physically moved, there will be little mixing between
the water in the sediments and that in the water mass above
the sediments.
This division of the water in an aquarium
into two discrete bodies of water, the water mass above
the sediments and the water mass in the sediments is
very important for the functionality of the sand beds and
aquaria. In the presence of bacteria, it results in the formation
of relatively discrete layers in the sediments based on the
diffusion of gases through the sediment water mass. These
layers are generally characterized by the concentration of
oxygen in the water, and they are classified as aerobic, anaerobic,
and anoxic. Aerobic layers have oxygen concentrations near
or at the level found in the free flowing water above the
sediments. Anaerobic layers have some oxygen present, but
the concentration is reduced from that found in the overlying
waters. Anoxic layers have no free dissolved oxygen, and may
be also referred to as reducing, as opposed to oxidizing,
layers.
If there was no life in the sediments,
there would be no layering. The layers are caused by the action
of bacteria, micro-organisms, and animals which live on the
sediment particle surfaces, and between the sediment grains.
As these organisms metabolize, they use up the available dissolved
oxygen. All of the oxygen in the sediments is consumed relatively
rapidly, resulting in anoxic layers, wherein the only life
is bacterial. Oxygen diffuses into the sediments from the
water above the sediments, but such diffusion is very slow.
In the absence of animals in the sediments, the aerobic and
anaerobic layers would each be a few hundredths of an inch
in thickness, and the anoxic layers would effectively extend
to the surface. Such layering is found in highly organically
enriched areas or in areas with toxic materials in the sediments.
In these areas, animal life is absent from the sediments.
These areas generally are polluted areas, but they don't have
to be; there are naturally occurring areas that mimic mankind's
best (worst?) efforts at pollution.
Here are some images of bacterial mats
on natural anoxic sediment surfaces: http://www.geomar.de/projekte/komex/gallery3.html
Organisms and Sediments
Because organisms live on and between sediment
particles, the interactions between those various organisms
are what makes sand beds so important in reef aquaria. Bacteria,
some microalgae, protozoans, and a few animals are small enough
to live upon sand grains. To these organisms, the sediment
bed, as such, does not exist; rather their whole world is
quite literally a grain of sand. On this super small scale,
the food web starts with the bacteria and microalgae, and
on this scale, the microalgae are predominantly cyanobacteria
and diatoms. These organisms live by absorbing dissolved materials
in the water around them and by metabolizing those nutrients,
creating more bacteria, cyanobacteria, and diatoms. Both the
bacteria and cyanobacteria will also actively secrete enzymes
into the surrounding environment, and these enzymes will breakdown
organic particulate material so that it may be absorbed. There
is sufficient light in all aquarium sediments for some photosynthesis
to occur; light sufficient for photosynthesis generally can
penetrate several inches or more into these sediments. The
relative proportions and types of bacteria and algae in the
sediments will depend upon the depth in the sediments, and
the relative amount of dissolved oxygen available. In the
upper layers of the sediments, diatoms and aerobic bacteria
predominate on the sediment particles. In the anoxic lower
regions of a deep sand bed, anaerobic bacteria predominate.
In between, there is a transitional mix of several types of
organisms, depending on the amount of nutrient, sediment disturbance
and water movement.
 |
|
Figure
4. Shallow water sediment surface from a Caribbean
coral reef. The sponges were attached to shell fragments
under the sediment surface. Note the brittle star arm,
it is about an inch long, but otherwise similar to the
small brittle stars found in reef aquaria. It moves
food from the water column into the sediments where
further processing of the food occurs. Note as well
the diversity of shapes and sizes of particles seen
on the surface. This sediment was near shore and very
poorly sorted.
|
Although these minute organisms are simultaneously
the ultimate consumers of dissolved nutrients, the aquarium's
biological filter and the source of food for other organisms,
they are but one part of complex web of interdependent sediment
organisms. This web is dependent upon the shallow-sediment-dwelling
animals for its existence and functionality. These most important
animals are the various sediment worms, snails, and crustaceans
that many aquarists refer to as "the clean-up crew."
It is important to realize that the diversity
of this group of organisms is really the cause of its utility.
Very few marine animals are omnivores, or eaters of everything.
Rather, they all tend to specialize on one kind of food or
another. Consequently, to make certain that all kinds of excess
foods are "disposed of," aquarists need to ensure
a rich and diverse sediment fauna.
 |
|
Figure
5. A small scale worm, about one half inch long,
crawling on the surface of a temperate sand bed. Note
the anemone burrow. Burrowing anemones are common in
both temperate and tropical sand beds, and pump much
water into and out of the bed as they expand and contract.
In reef aquaria with sand beds, other animals generally
serve the same purpose as these small anemones are seldom
sold in the hobby.
|
At any level in a food web or any link
in a food chain, most of the food that is eaten is not assimilated
into the tissue of the animal doing the eating. Generally,
as an ecological rule of thumb, only about ten percent of
the food eaten by any animal stays in that animal as part
of its tissue. Some of the remaining ninety percent of the
eaten food is burnt as fuel in respiration to provide energy
for the organism. Burnt fuel exits the animal as water and
carbon dioxide and this eventually leaves the aquarium. A
lot of food is "spent" this way; so much
food is converted into carbon dioxide in every aquarium every
night that the carbonic acid produced by this exhalation will
significantly lower the pH of the system. Additionally, some
of the food is used in other metabolic functions, and the
byproduct of this is the waste ammonia and phosphates excreted
by the animal through its urine, or simply across its body
surface. However, that is still only a small part of the food.
The majority of the unassimilated food is passed out of the
digestive tract as feces. Fecal matter in marine ecosystems
is simply indigestible or undigested foods mixed with some
digestive enzymes and intestinal bacteria. As unappetizing
as this stuff may sound, it is a major food source for much
of the fauna of a coral reef, including corals, and fishes
such as clown fishes (See Hamner, et al, 1988 for a discussion
of just how much "coprophagy" (or eating of feces)
is a part of the reef).
Aquarists feed their systems to keep their
decorative animals in good health. The amount of food necessary
to maintain a large well-stocked aquarium is quite significant.
However, most of that food is not used by the organisms that
it is meant for, it either is either converted into dissolved
nutrients or it is converted into feces. Both of these materials
must be removed from the aquarium or converted into some harmless
product. That conversion is almost entirely the done in the
sediments, and it is done by cycling food over and over through
various animals and microbes until there is either no nutritional
value left in it or it has been totally converted to soluble
gases that leave the system.
This process begins in the uppermost sediments
where carrion-feeding animals such as the small fireworms,
Linopherus, and snails, such as Nassarius, eat
excess meaty foods such as dead brine shrimp or flake food
rich in meat byproducts. Other animals in the uppermost sediments
eat "vegetable" material. In natural systems, this
vegetable material would be primarily algal remnants or the
remains of sea grasses. In aquaria algal remnants are present,
but so are vegetable byproducts in flake foods. In most aquaria
the animals that eat this material are amphipods, some semi-omnivorous
snails such as the temperate Illynassa obsoleta, and
surface grazers such as the conchs, Strombus species,
and mopping sea cucumbers.
One aspect of all of these animals living
and feeding in the sediments at the surface is that a lot
of dissolved nutrients are excreted by these animals into
the sediments. These nutrients will, in their turn,
go to fuel algal growth on the sediments. In aquaria,
these algae are predominantly diatoms and the photosynthetic
bacteria called cyanobacteria. An interesting small
sub-cycle of nutrient utilization occurs where algae are:
-
eaten by the various grazers, including
at this level, the small harpacticoid copepods, and the
small seed shrimps or ostracods, which scrape microalgae
off of the individual sand grains,
-
processed through their metabolism
resulting in,
-
part of the algal mass being assimilated
by the grazers,
-
part being respired, and
-
part being excreted as dissolved nutrient
to fuel more algal growth.
Of course, with each pass through the cycle,
the amount of nutrient available for algal growth would decrease.
Or, it would if no more was being added by feeding.
But, of course, more food always has to enter the system.
Nevertheless, this algae-grazer nutrient cycling process does
go a long way to remove a lot of excess food from the system.
However, not all of the food eaten by the
surface grazers remains on the surface; many of the surface
grazers will dive under the sediment surface as soon as they
have eaten, and digestion will occur in the relative safety
of the substrate. Excretion of the various wastes occurs at
varying depths below the surface. Additionally, other animals
such as the tube-dwelling, suspension-feeding Phyllochaetopterus
worms, or the suspension-feeding small brittle stars, add
both dissolved wastes and feces below the sediment surface.
These worms eat small particulate material in the water, and
should therefore also be considered to be a part of the clean-up
crew. Basically, these suspension-feeding animals are living
mechanical filters. Other subsurface worms that may feed upon
surface particulate material, such as the cirratulid hair
worms and the tube dwelling spaghetti worms, do much the same
thing. In effect, all of these worms move material from the
surface and deposit it some distance down into the sediments.
 |
Figure
6. Small surface deposit feeding tube worms called
"oweniids." These worms are common in some
tropical habitats, such as sea grass beds, and feed
by "daubing" the surface for food. As they
move up and down in their tubes, in a manner similar
to tube worms in aquaria, they pump water in and out
of the sediments.
|
Such material still is food and, of course,
there are yet more worms and other animals that process it.
One thing that is often overlooked, in discussions of food
transfers such as this, is an interesting reversal of a trend
seen above the sediment surface. Each of these food transfers
corresponds to going one more link or level up a food chain
or web. When this occurs in the water column or on land, the
animal that is that link gets eaten by yet another larger
animal. The final animal in the food chain is generally the
biggest critter around. In these sediment-based systems, the
animals of each succeeding level are generally smaller than
those of the preceding layer. Although there are subsurface
predators in the sediments, they are limited in size by the
sediment properties and prey sizes. The largest wholly infaunal
predatory animals are worms and are generally no more than
a foot or so long, and they are almost never found in aquaria.
Nonetheless, the sediments in the lower
part of the aerobic layer and upper anaerobic areas are a
busy place. In addition to the surface feeding animals discussed
above, there are animals here that are only found under the
sediment surfaces. These include some of the nematodes or
round worms. This is a diverse group containing both herbivores
and carnivores; nonetheless, except for a few species, their
natural history and aquarium biology is effectively unknown.
Some of them will undoubtedly be eating small particulate
organic material, either worm feces, algal or bacterial clumps
or some other material. Others may eat small polychaete worms
or protozoans.
The subsurface community of organisms also
includes a rich array of protozoans. These include highly
mobile ciliates, some which look quite like flatworms, and
shelled but effectively immobile foraminiferans. All of these
are predators that graze upon bacteria, bacterial aggregates,
or algae. In turn, these algae and bacteria thrive in this
area because of the action of the surface feeders that pump
food and nutrients into this zone.
Flatworms are found throughout the upper
sediment layers, but are most commonly found within the sediments
near the lower boundary to the aerobic layers. Many of these
are predatory and eat copepods and small worms; others eat
the abundant microalgae in this area.
Some of the larger and most impressive
animals found wholly within the sediments of this level are
polychaete worms, such as the syllids. These worms will reach
lengths of an inch or more. The ones I have seen appear to
subsist, depending upon the species, on other polychaete worms,
or bacterial aggregates.
One other byproduct of the animals living
in the sediments needs to be addressed, as it is very important
to aquaria. This particular product is produced in sand beds
where the animals are doing well, and that product is the
spawn from the animals in the bed. Once the bed animals are
thriving, they reproduce regularly, and this reproduction
is in the form of eggs, sperm, and larvae liberated at the
sediment surface into the overlying water. This material is,
of course, recycled food added to the aquarium some time before
hand, and it is now in the form where it is eminently good
food for many suspension-feeding animals in the system. So,
here again, nutrient has been moved back up out of the sediments
and into the water for corals and other animals to eat.
The functionality of these sediment layers,
in the context of either the aquarium or natural ecosystem,
is dependent upon the diversity and richness of organisms
in the sediments, and this is directly related to the sediment
particle distributions that were mentioned previously. Well
sorted sediments with a narrow particle size range, are generally
quite optimal for a few organisms, primarily those adapted
to that particle size range. For everybody else, well…
they don't work so well. In aquaria, where the maximum diversity
and richness is required, the aquarist needs to ensure that
the sediment particle size range is fairly large. Of course,
"fairly large" is a matter of opinion. Marine benthic
ecologists and other folks that study sediments categorize
sediments in a series of sizes based on the negative logarithm
to the base 2 of the size. Sounds pretty complicated, but
really isn't. What this means is that starting with the upper
sand limit of four millimeters in diameter, the sand size
categories are: 2 mm to 1 mm, 1 mm to 0.5 mm, 0.5 mm to 0.25
mm, 0.25 mm to 0.125 mm, and 0.125 mm to 0.063 mm.
|
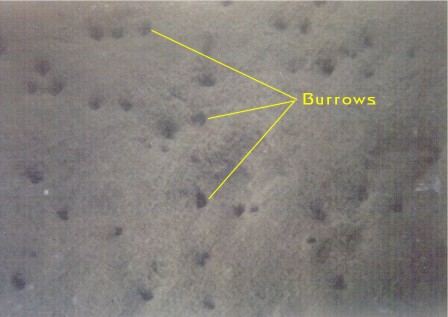
Figure
7. Silty sediments, where the sediment particles
are typically smaller than one-sixteenth millimeter
in diameter are common in lagoonal backwater areas protected
from wave action. These sediment areas are at the opposite
extreme of the sediment areas illustrated in Figure
1. Here, the environment is very stable and there are
multitudes of animal burrows. These sediment areas are
the "power houses" of nutrient processing
because of the high density of animals found in them.
|
 |
For a sand bed to contain the most animals
of the most species, it really should have a distribution
where sediment sizes span from about 2 mm to 0.063 mm (2 mm
to 1/16th mm), and where most of the particles are in the
0.250 mm to 0.125 mm range. This will make a sediment that
is acceptable, if not perfect, for most animals.
The animal life in the subsurface layers
is, of course, only a single component of the rich array of
organisms found in this level of sediments in our tanks. This
area is rich with various algae and bacterial species. Most
aquarists tend to think that most algae are something deleterious
and their presence in reef tanks is considered a problem.
However, most of the non-bacterial life on a "coral"
reef is algae. In fact, the biomass of algae on such a reef
is often on the order of five to ten times the biomass of
corals. The reality of the situation is that these reefs are
algal reefs, with a thin frosting of corals and other animals.
(Odum and Odum, 1955).
Since both the corals and the algae thrive
under the same environmental conditions, and since it is therefore
impossible to keep algae out of reef tanks, it is best to
manage the tanks so that the algae are beneficial. The sand
bed subsurface algae definitely are beneficial. They utilize
dissolved nutrients and, in turn, provide nutrition for many
of the small animals found in these layers. A similar situation
is seen with bacteria, they also utilize nutrients and are
food for deposit-feeding animals. Animals that feed on sediments
are not feeding on the mineral grains of the sediments but
rather are consuming the bacteria and algae adhering to those
sediment particles.
By now, the basic properties of all of
these cycles should be obvious. Dissolved nutrient is utilized
by the algae and bacteria to produce more bacteria and algae.
In doing so, it is removed from solution, and some of it is
respired away as dissolved gas. The gas eventually leaves
the aquarium. In turn, the algae and bacteria are eaten by
some animal, and some more of the once dissolved nutrient
is respired away. Some of the once dissolved nutrient is incorporated
into the predator and some is once more released as dissolved
nutrient in the predator's urine. Take a look at the
Publix Ad for Halloween. Generally, if a piece of
food drops to the bottom of an aquarium (or the ocean), enough
energy (in the form of sugars or carbohydrates) is in it to
provide fuel for five to six cycles through decomposers and
detritivores.
Each of these cycles progressively removes
some of the useful energy and materials from each bit of food,
until all that remains are materials that are insoluble or
materials locked up in some organism. Such a continual cyclic
process can remove an amazing amount of material from an aquarium,
but it can't remove all of it, by itself. The single most
critical factor in all of these processes is the transfer
of materials from one state to the next, whether it is from
one organism to another by feeding, or by passing from nutrient
to organism. Each time such a change occurs, energy is used
up and materials are respired away.
The key to the success of such a sand bed
community is water movement between the sediment grains. I
mentioned above that it is essentially impossible for waves
or water currents to move water in sediments. However, there
is an exceptionally useful method of generating slow and even
water movement through sediments. This water movement is caused
by the motion of the animals in the upper inch or so of sand,
particularly in those vertically-oriented tube worms such
as Phyllochaetopterus, but also by all other animals
moving in the upper sediment layers. The amount of water moved
by one worm is quite small, on the order of a few fractions
of a milliliter per day to a couple of milliliters per hour,
but the cumulative total of all the water moved by
all the animals in the sand bed is quite considerable.
It is enough to push water into and through the sediments.
Additionally, it has been estimated that
each small animal over the course of a day disturbs around
a hundred cubic millimeters of sediment. A hundred cubic millimeters
is not very much, but when multiplied by the number of animals
in a sand bed… well, the bed positively vibrates. In
my 45 gallon lagoonal reef, by doing sediment samples and
counting the number of animals in the sands, I estimated that
there were between 90,000 and 150,000 animals in the sand
bed with a foot print of about three feet long and one foot
wide. Such a population density translates into about 300,000
to 450,000 animals per square meter, a value quite consistent
with values found in rich sand or sandy mud ecosystems in
nature. There was enough activity in the tank to move virtually
all the sand in the tank every few days. Of course, it doesn't
really occur that way; most of the motion is limited to the
upper layer where it facilitates water movement. Proper functionality
of the lower parts of the sand bed require no disturbance
except the gentle, and slow, movement of water through them.
In other words, in the sand bed of a normal
reef tank, there is the capability of having a sediment community
with a population comparable to natural systems. Such a bed
functions like a natural system as well. It metabolizes and
uses organic materials moving excess materials through food
webs and chains, and allowing their export from the system.
Not everything will leave the system, however,
and what remains also follows a pattern seen in natural systems.
Only a few gaseous materials will exit the system as respiration
byproducts. Other soluble materials will accumulate in the
system's water and will have to be removed by skimming. Still
others, particularly the toxic materials, will tend to get
concentrated in animals or precipitated as insoluble minerals
by bacteria. The slow water movement caused by the action
of the upper sand surface worms pumps water slowly through
the lower anoxic regions of the sand bed. Here, bacteria and
chemistry combine to produce conditions that result in the
precipitation of many toxic heavy metals such as sulphide
and iron hydroxide minerals. (Pincher, et al., 1999, 2000)
Such materials accumulate in the tank with time, but as long
as these sediments remain anoxic, those poisons are locked
there and can be considered "safe."
Conclusion:
By simply setting up a deep sand bed, and
then maintaining that bed with the proper diversity and mix
of animals, reef aquarists can facilitate the utilization
of the necessary excess nutrient resulting from normal feeding.
Such beds also efficiently, but slowly, detoxify toxic trace
metals. The large populations of sediment animals also transfer
nutrients from the sand bed back to organisms such as corals
and soft corals by their action of moving sediments and water
which generates bacterial particulates in the tank water mass.
Finally, as these small animals reproduce they also transfer
excess nutrients back from the sediments into the water mass
in the form of larvae and reproductive products.
|