|
Introduction
One of the most confusing aspects of reef
aquariums is the subject of lighting. Much of the problem
stems from an insufficient understanding of the basic properties
of light. The purpose of this article is to shed some light,
so to speak, on light and its properties in the hope of improving
aquarists’ understanding of this apparently complicated
topic. This article is only meant to be an introduction to
the properties of light. More detailed information is available
from a good physics textbook.
This first article will cover some of the
physical properties of light that have more relevance to reef
aquariums.
What is Light?
Light, particularly that from the sun,
is one of the most important factors affecting the earth.
Almost all life on earth relies directly or indirectly on
light (and heat) from the sun. But what is light?
Light is a form of electromagnetic radiation,
and includes visible light (and by some definitions) ultraviolet
and infrared radiation. Other forms of electromagnetic radiation
include gamma rays, x-rays, microwaves and radio waves. Light
exhibits both particle-like and wavelike behaviours. The "particles"
(while not truly particles) are bundles of electromagnetic
energy called photons. A photon is defined as a quantum of
electromagnetic radiation. They have zero mass, no electrical
charge and an indefinitely long lifetime. Photons always travel
in straight lines, unless under the influence of gravity.
The effect of gravity is so small that for practical purposes
we can assume light travels in a straight line.
Ignoring amplitude (height), waves have
two important characteristics: frequency and wavelength and
these are in a reciprocal relationship. Increase the frequency
and the wavelength gets smaller. Decrease the frequency and
the wavelength increases. Waves on the ocean also exhibit
this characteristic. The wavelength is the distance between
the wave peaks and the frequency is the number of waves peaks
that pass in a certain period of time.
While light is not truly made of waves,
it can behave like it is. Light may be described as being
composed of different wavelengths and frequencies. Light at
a wavelength of 555 nm has a frequency of 540 x 1012 hertz.
The energy of the photon is based on its frequency. The shorter
the wavelength, the higher the frequency and the greater the
energy of the photon.
The perceived colour of light is due to
energy of the photons. Photoreceptors respond to a narrow
range of energy. Energy within the range correctly stimulates
the receptor and we perceive that as colour. As the energy
level of the photons is based on their frequency and wavelength,
colour of light is normally discussed using its wavelength.
Light with wavelengths between 400 nm (nanometres) and 700
nm is visible (to human eyes). Light with a wavelength around
400 nm is perceived as blue and at around 700 nm it is perceived
as red. Below 380 nm (down to around 100 nm) is ultraviolet
radiation. Infrared radiation (which we perceive as heat)
ranges from 750 nm to 2500 nm. In the same way as our eyes
react to different wavelengths of light, the pigments in plants
and algae react to certain frequencies differently than to
other frequencies. Chlorophyll-a, for example, has absorption
peaks at around 440 nm and 650 nm.
Photons may be reflected and they may be
refracted by passing at angles through objects of different
densities. Light travels at 3 x 1010 cm.s-1 through a vacuum
and nearly as fast through air, however, its velocity is greatly
reduced in dense media such as water or glass.
Reflection and Refraction
When light reflects off a mirror or similar
surface, the rays reflecting off the surface will exit at
the same angle on the other side of line perpendicular to
the surface as the incident rays. This is the law of reflection.

Figure 1: Law of Reflection.
The angle of reflection equals the angle of incidence.
This type of reflection, where the majority
of the rays follow the law of reflection, is known and specular
reflection, and is what you normally see from polished aluminium
reflectors in lamps. If the surface is particulate, most light
rates do not follow the law of reflection and instead are
reflected in multiple directions. This is called diffuse reflection
as is the characteristic of most painted surfaces. Light reflected
off these surfaces appears uniformly bright regardless of
the angle of view.
When light passes through materials of
different densities, the velocity of the light changes slightly
and this causes a bend in the ray at the interface between
the two materials. This is known as refraction and is the
principle behind lenses and is also why objects appear larger
when viewed through a face mask underwater. Refraction is
dependent on the differences in densities of the two materials,
also called refractive index, and the angle of incidence.
Perpendicular rays are not refracted, but as the angle of
incidence increases so does the refraction.
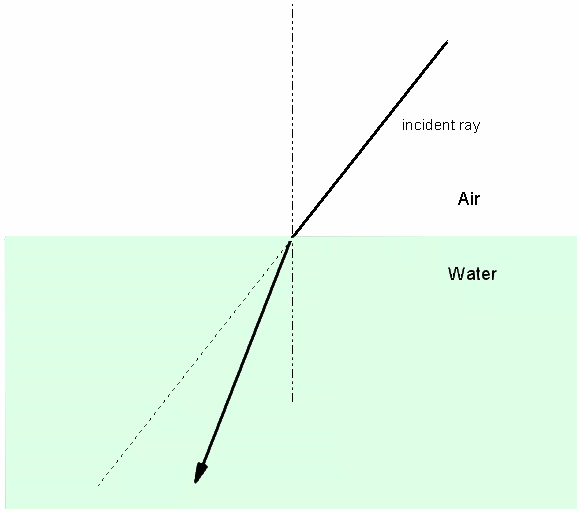
Figure 2: Refraction. An example of
refraction caused when light
traveling through air hits the water surface at an angle.
Light Intensity
The intensity of light is one of the most
important aspects of light relative to photosynthetic organisms.
The more intense the light, the more energy is available for
photosynthesis. Basically, intensity is the number of photons
hitting an area over time.
First, let’s define some units. Don't worry too much
about the units themselves, it is the principles that are
behind them that are important.
A lumen is a measure of the power of visible
light. One lumen is defined as the luminous flux of 1/683
watt (see sidebar) of light at 555 nm. However, a lumen does
not measure intensity. It is generally used to measure light
output. For example, a 36W fluorescent tube radiates a total
of 3250 lumens, but this is the total amount of light being
radiated in all directions.
A lux is a measure of illuminance. One
lux is defined as the intensity of luminous flux hitting a
surface at 1 lumen/square metre. The intensity of the light
is dependent on the total amount of luminous flux and the
area over which it is spread. The intensity is just how much
light reaches a surface. The illuminance of the sun at noon
at the equator is over 100,000 lux.
One problem with both lumen and lux is
they are weighted to match the human responsiveness to light.
Therefore, yellow-green may be overstated, red and particularly
blue will be understated.
Photons can be counted (using a quantum
meter) and are reported as Einsteins. One Einstein is one
mole (see sidebar) of photons. For measuring light intensity,
the number of Einsteins hitting a area over time are measured.
This is usually seen as E.m-2.s-1 or uE.m-2.s-1. Photosynthetically
Available Radiation (PAR) measures all visible light (400
to 700 nm) fairly uniformly and is reported in Einstiens per
square metre per second (E.m-2.s-1). In tropical latitudes
around noon on a day with no cloud, the average PAR at the
sea surface is around 2.5 E.m-2.s-1 (Tomascik et al 1997).
It is important to understand the difference
between the light output of a light source and the intensity
of the light reaching a subject. It is time for an analogy.
If you bought a large bag of sand and placed it in a tank,
spreading it evenly on the bottom of the tank, it would be
at a certain depth. The total light output of a lamp is equivalent
to the volume of sand. The light intensity is the depth of
sand. Place the same volume of sand in a smaller tank and
the sand would be deeper. Use a larger bag of sand (a brighter
lamp) and you will get more depth.
A number of factors affect how much light
radiated from a light source actually reaches the subject.
The most important factor affecting the light intensity is
the distance between the light source and the subject. The
rays of light from a point light source are divergent and
so the light is spread over a larger area as the subject moves
away from the light source. The loss of intensity due to distance
is predictable and is known as the inverse square rule. The
inverse square rule states that the light intensity will be
in inverse proportion to the square of the distance from the
light source. That is, if you double the distance from the
light source, the intensity will be reduced to 25%. Figure
1 shows the inverse square rule in practice.
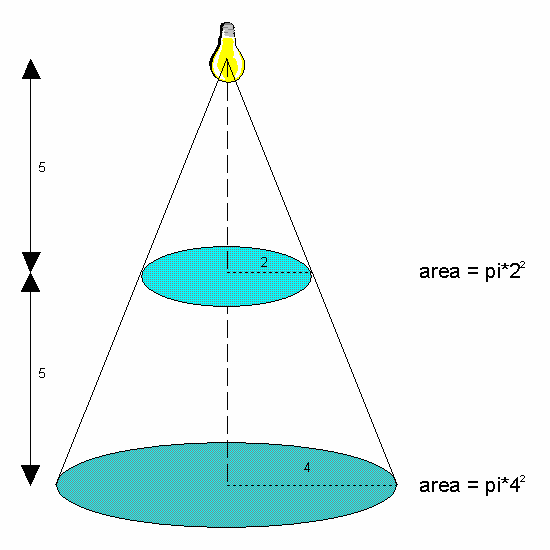
Figure 3: Inverse Square Rule.
At 5 units from the light source, the
light is spread over an area of pi*22. At 10 units from the
light source, the same light is now spread over an area of
pi*42. The light intensity at any point on the lower circle
will be one quarter of that at any point on the higher circle.
The inverse square rule holds for any light
source that approximates to a point and will hold whenever
the distance from the light source is more than 5 times the
largest diameter of the light source. The sun approximates
to a point light source. As it has a diameter of around 1.4
million kilometres and is at a distance of 150 million kilometres,
the inverse square rule applies. Of course, the size of the
earth prevents us from moving to distances closer to or further
from the sun to see the inverse square rule in action. Incandescent
and metal halide lamps approximate to point light sources
as do some compact fluorescent lamps. Regular fluorescent
tubes do not approximate to a point light source over the
distances we use them in our aquariums.
The Colour of Light
As explained above, the perception of colour
is based on the energy level of the photons reaching the photoreceptors
in our retinas. The energy level is related to the wavelength
of light and so wavelengths (in nanometres) are used to describe
the colour of light. These are the approximate wavelength
ranges for the colours we perceive:
| Colour Wavelength |
(nm)
|
| Violet |
390-450 |
| Blue
|
450-490 |
| Green |
490-570 |
| Yellow |
570-590 |
| Orange |
590-620 |
| Red |
620-770 |
What we see as "white" light
is actually a combination of these wavelengths. If "white"
light is projected through a prism, the component wavelengths
are split out due to slight variation in velocity of the different
wavelengths as they pass through the more dense prism. This
is also what causes a rainbow. Sunlight has what is known
as a continuous spectrum. It contains a continuous range of
wavelengths from below 400 nm to above 700 nm.
The perceived colour of artificial light
is based on the relative intensities of the component wavelengths.
Many lamp manufacturers publish the spectrums of their lights.
Most artificial light sources produce light with an interrupted
spectrum. The light is made up of a number of different wavelengths,
but not all wavelengths are represented.
Absorption of Light
Water absorbs light and even in clear water
about 60 percent of total radiation entering the water is
absorbed in the first metre, and around 80 percent is absorbed
in the first 10 metres (Gross 1977). Additionally, 3 to 50
percent of incident light is reflected off the water surface
depending on the angle of incidence (Tait 1972). At noon,
the angle of incidence is small and there is little reflection,
but early and late in the day and angle is much greater and
much of the light is reflected. Turbid water absorbs and reflects
more incident light resulting in even greater attenuation
of light.
Water absorbs different wavelengths
at different rates. Red light is absorbed by water very quickly
and even at a depth of 3m there is a significant loss of red
wavelengths. Blue light, however, is absorbed much more slowly
and much of the blue light hitting the water surface penetrates
to 40m or more. Similarly, UV-A radiation has been shown to
penetrate 20m of water or more.
The differential absorption of the wavelengths
significantly affects the colour of the light reaching all
but shallow depths. Ocean water far from the coast is usually
very clean and has few coloured particles or dissolved substances.
This water appears deep blue as a result of the scattering
of light rays in the water (Gross, 1977).
The next two properties, colour temperature
and colour rendition index, are more methods of describing
light, particularly artificial light sources, rather than
actual physical properties of light.
Colour Temperature
If you turn on an electric stove element
you will notice that it radiates both heat and light - it
glows. The hotter the element, the brighter it glows. At the
range of temperatures you can get from a stove, the colour
of the radiated light is red. If you were able to heat up
the element further, the colour would change, first becoming
orange, then more yellow and eventually what we see as "white"
light. This is the principle behind colour temperature.
Colour temperature is based on radiation
from a theoretical black body rather than a stove element.
As the black body is (theoretically) heated, the colour of
the light radiated shifts from the red end (longer wavelength,
less energy) to the blue end of the spectrum (shorter wavelength,
more energy). The colour temperature of the light produced
by the black body is actually the temperature of the body
in Kelvin (see sidebar).
The colour temperature really describes
the spectrum of the light and the relative quantities of different
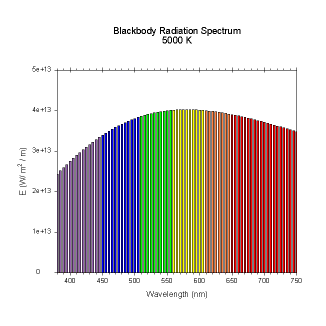
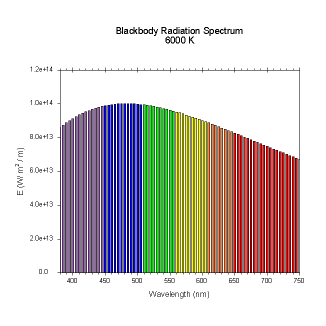
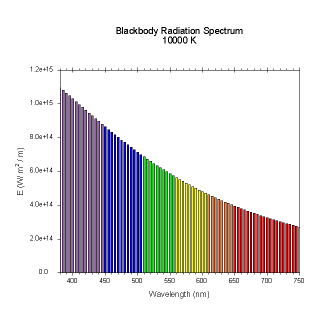
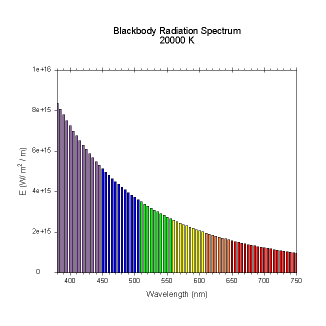
wavelengths. Here are the black body radiation spectrums for
a number of different temperatures (courtesy of Dallas Warren):
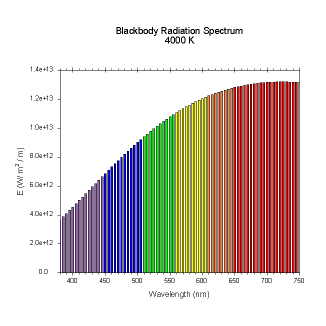 |
 |
|
Figure 4
|
Figure 5
|
 |
 |
|
Figure 6
|
Figure 7
|
 |
 |
|
Figure 8
|
Figure 9
|
Figures 4-9. Black
body radiation. Example spectrums of black body
radiation at 4000K, 5000K, 6000K, 7000K, 10000K and 20000K.
Not all light sources necessarily follow
the characteristics of the theoretical black body. Our sun,
however, is a pretty good match. The Sun itself produces light
with a colour temperature at around 5800 K, however, as light
from the Sun gets reflected and refracted by the earth's atmosphere,
the actual colour temperature of the Sun will vary with different
conditions. At noon, on a clear day, the direct light from
the Sun alone is around 5500 K, but with the light from the
sky included, it is around 6500 K. For this reason 'Daylight'
is usually defined as 6500 K. At noon, on a clear day, in
shade (so there is no direct light from the sun), the colour
temperature may be higher than 20000 K.
Standard incandescent lamps also fairly
closely follow the theoretical black body and this is mainly
due to the fact that incandescent light produce their light
by heating a filament. An incandescent lamp has a colour temperature
of around 2300 K.
Fluorescent and gas discharge (e.g. Metal
Halide) lamps do not follow the theoretical black body and
the rated colour temperature is only an approximation of the
colour of the light produced. This is largely because these
lamps produce an interrupted spectrum with peaks in some wavelengths
while some wavelengths are not radiated at all. However, lamp
manufacturers will still publish colour temperature information
for their lamps which would be more accurately termed "apparent
colour temperature".
Please note that colour temperature can
only be applied to "white" light, that is, light
that has a mixture of all wavelengths. Actinic lights, for
example, do not have a colour temperature as such.
Colour Rendition Index (CRI)
Artificial light sources are also rated
with a Colour Rendition Index. This is a indicator of how
well colours will be rendered under that light source. A CRI
of 100 means colours will be rendered as well as they are
under sunlight at noon. Smaller numbers mean the colours will
not be rendered accurately. The closer the index is to 100,
the more accurate the colours will appear.
In my next article I will cover the biological
aspects of light.
|