|
In a recent article,1
Ron Shimek showed that two salt mixes and natural seawater
were more conducive to the development of sea urchin embryos
than were two other salt mixes and the water from two reef
aquaria using one of them. In that article, Shimek suggested
that elevated metal levels, such as copper, could have been
responsible. The two salt mixes with poor development are
reported to have higher levels of a number of potentially
toxic metals than the other two salt mixes or natural seawater.
While there may be other explanations for
the observed differences, including ammonia, nitrite, pH,
organic compounds present intentionally or as impurities,
and the elevation or depletion of many different inorganic
chemicals, the hypothesis concerning toxic metals is quite
reasonable. As a result of that article, as well as earlier
ones that showed the elevated levels of certain metals in
reef aquaria and salt mixes,2,3
many aquarists have become interested in finding out what
they can do to lower the toxic metals concentrations in their
aquaria. This article starts with the premise that toxic metals
are responsible for the biological effects that Shimek observed,
and explores what options aquarists have to solve the problem.
Some have assumed, seemingly incorrectly,
that if they use natural seawater or one of the salt mixes
shown to have lower metals, that the problem will be solved.
Unfortunately, some of the things that aquarists need to do
in maintaining reef aquaria may well be a primary source of
metals. Specifically, the foods and calcium and alkalinity
supplements that aquarists use are a big source of metals
to reef aquaria. The complication of these inputs may make
it difficult, if not impossible, to maintain an aquarium with
natural levels of these metals. Both foods and supplements
have enough metals in them to take natural seawater to the
levels found in the "high metals" salt mixes in
a fairly short period of time. In evidence of this possibility,
the one aquarium in Shimek's study of reef aquaria that used
natural seawater had amongst the highest levels of many of
these toxic metals.2 Habib
Sekha has also indicated that he has obtained similar results
on two other aquaria using natural seawater (pers. comm.).
That is, the copper and nickel levels were equivalent to those
levels found by Shimek in aquaria using artificial salt mixes
such as Instant Ocean.2
The copper levels in these three aquaria using natural seawater
are also comparable to the levels found in my own tank using
only Instant Ocean salt mix (10-13 ppb copper).
Additionally, one would expect that the
total input of these different metals would be very different
between reef aquaria. And yet, all of the aquaria in the study
mentioned above had copper concentrations within a factor
of two of each other.2 That
suggests to me that the controlling factor is more likely
to be export than import, and this possibility and how it
impacts the actions of aquarists wanting to maintain aquaria
with low metals is discussed at the end of this article.
In this article, I will focus on those
potentially toxic metals that have been found to be elevated
in operating reef aquaria,2
including copper, nickel, zinc, molybdenum, and cobalt. I
will also use the premise that aquarists want to maintain
an aquarium with a lower concentration of such potentially
toxic metals, and will provide some guidance and general information
on how aquarists might usefully go about operating aquaria
in that fashion. In some cases, these techniques will relate
to many metals, and in some cases they will only apply to
certain metals, such as copper.
Using methods described in this article,
and other articles to follow that will attempt to further
quantify how effective these suggestions are, some aquarists
can potentially begin to operate aquaria with much lower levels
of such metals. Whether such a reduction will have important
advantages that aquarists will appreciate can only be demonstrated
when reef aquaria are operated under such low metal concentrations
and compared to reef aquaria operated in more traditional
means. Once that has been accomplished, then aquarists will
be able to look at the results and determine for themselves
if this is a necessary, or at least a prudent action for them
to adopt.
To begin, however, it is important to
note that there are no simple solutions to this potential
concern. The two obvious ways to consider lowering the metals
concentrations in aquaria are to add less, and export more.
There are things that aquarists can do to immediately reduce
additions of toxic metals, and these will be detailed in this
article. Whether the "normal" export systems (skimming,
growth and harvesting of macroalgae, activated carbon, etc.)
can be useful may in part depend on how much of an input load
for which they are expected to compensate. Other "chemical
filtration" systems have been promoted to hobbyists for
this purpose, but in the end may not be successful in reducing
metals levels below those already present in many aquaria,
regardless of the inputs. Still other approaches to export,
such as binding metals to calcium carbonate media, are more
speculative and will be mentioned as suggestions that will
be reported on in more detail in future articles.
In order to understand how and why certain
methods of dealing with metals may work (or not), we also
need to know the nature of these metals in aquaria. Many aquarists
will naturally think of these metals as being like the more
familiar calcium ion, floating free through the water on its
own. The true situation for many of these metals is, however,
much more complicated. It is expected that more than 99.9%
of the copper ions present in the water column of reef aquaria
are attached to something else, and what they are bound to
has a big impact on how they behave. Consequently, before
getting into metal removal methods, this article will describe
what forms the metals take in seawater, and what forms they
are expected to take in reef aquaria.
Metals in Seawater and Aquaria
Different metals take different forms in
seawater. Some exist primarily as free metal ions in solution.
Of those that aquarists are most familiar with, sodium (Na+),
potassium (K+), calcium (Ca++) and magnesium
(Mg++) fall into this category.4
All of these may, to some extent, form complexes with other
ions and organic materials, but the free ion tends to predominate
in seawater and in typical aquaria.
The metals that we are most concerned about
in this article, however, have much more complex behavior.
Copper, for example, exists in a multitude of forms.4
In natural seawater, it has recently become clear that copper
is almost completely bound by organic materials.5
Many of these organics are called chelators. A chelating agent
is one that can grab onto the copper from two or more directions
at once.
In natural seawater, these organics take
many forms. Humic and fulvic acids, for example, are two of
the most important types of materials that bind copper and
other metals in seawater.6
They are also known to greatly reduce the toxicity of metals
because in many cases it is the free copper that is the most
toxic.5 These classes of
organic materials comprise what remains when proteins, carbohydrates,
and many other naturally occurring organic materials are biodegraded
to a state where further degradation is very slow. Humic and
fulvic acids (the distinction between the two being just that
humic acids are more hydrophobic than fulvic acids) have a
wide range of structures and physical properties. They typically
are high molecular weight organic acids, with sizes ranging
from 500 to 10,000 daltons (grams/mole). They can also be
parts of larger assemblies of organic materials that would
be called colloidal (very small particulates) rather than
"dissolved". The humic and fulvic acids are comprised
of amino acids, sugars, amino sugars, fatty acids, and other
organic functional groups. Different localities and depths
in the ocean have different amounts and specific types of
these organic materials present. Typical values for the total
dissolved organic carbon are on the order of 1 ppm carbon
for tropical surface seawater.6
Humic substances typically account for about 10-20 % of that
total, and fulvic substances can account for more than 50%.6
Within these structures will be sites where
several carboxylic acid, phenolate, thiolate, amino, or other
metal-binding groups come together. These sites are where
a metal ion will be most strongly bound. Structurally, it
is hard to show a "typical" humic acid binding to
copper, but the structure below shows one possibility:
Figure 1. A schematic of a copper ion (Cu++;
shown in red) being
chelated by a naturally occurring humic acid (shown in green).
In this figure, the central positively-charged
copper ion (Cu++) is chelated by the larger humic
acid shown in green. It is bound ionically by two negatively
charged carboxylic acid groups and complexed by one neutral
amino group. Together these three groups may hold the copper
ion more strongly by many orders of magnitude more strongly
than could any individual binding group.
In the very extensive book "Biogeochemistry
of Marine Dissolved Organic Matter",6
it is stated:
"It is now widely accepted that the chemical speciation
of most bioactive metals in seawater is regulated by strong
complexation with natural organic chelators…The cycling
of bioactive metals therefor is intrinsic to the behavior
of this subset of organic constituents."
And also:
"The collective findings establish that a significant
component of bioactive, or nutrient, metals (Mn, Fe, Co, Ni,
Cu, Zn, Cd) occur in the colloidal phase along with numerous
other trace metals."
Free Metal Ions
In seawater, copper is expected to be bound
to organic materials. In one recent study of copper in natural
seawater, more than 99.97% was bound to organic materials.5
Other metals, such as zinc, may not be as extensively chelated.
In aquarium water, where the level of both metals and organics
can be higher than in seawater, the percentage bound to organics
may be even greater. Nevertheless, unchelated metals are very
important. In the case of copper, for example, the unchelated
copper ions may represent that portion of the total copper
that is toxic to many organisms.5
These inorganic forms of copper and other metals are also
expected to predominate in freshly mixed artificial salt water
that has not been exposed to sources of organic materials.
Assuming that the organism does not take
up the entire organic molecule to which the metal is attached
(and many humic acids are known to be poorly taken up due
to their refractory chemical nature6),
then chelated metals are often much less toxic than unchelated
metals. In seawater, for example, the speciation of copper
(i.e., whether and how it is chelated) is often much more
important for understanding overall toxicity than is the total
copper concentration.7
The portions of metals in seawater that
are not bound to organic materials are very complicated in
their own right. Copper, for example, takes at least 7 different
soluble inorganic forms in seawater.4
It is comprised of Cu++ (3.9% of the inorganic
copper), CuOH+ (4.9%), Cu(OH)2
(2.2%), CuSO4 (1%), CuCO3
(73.8%), Cu(CO3)2--
(14.2%) and Cu(HCO3-
)+ (0.1%). Similar complications hold for many
of the metals that we are concerned with in this article.4
Speciation and its Effect on Binding Metals in
Aquaria
Since these metals take many different
forms in aquaria, one must consider the nature of these different
forms when developing methods for removing them. For example,
metal ions such as Cu++
or Ni++ will never absorb
at the air/water interface to permit selective removal by
skimming. However, if the same metals were bound to an organic
material that itself adsorbed to the air/water interface,
the metals might well be exported by skimming. Similar concerns
relate to claims about metal removal using activated carbon,
polymeric ion exchange and complexation resins, and binding
to inorganic materials like iron oxides and calcium carbonate.
In fact, any proposed method of metal removal will be significantly
impacted by the nature of the metal speciation. Depending
on what is added to any particular aquarium, the speciation
may actually vary from aquarium to aquarium, potentially making
generalizations about them less useful.
Further, any experiment that purports to
show how well something works must be carried out under conditions
of the real speciation present in aquaria. Tests run in artificial
salt water (or worse yet, fresh water) are not necessarily
of any use in predicting efficacy of metal removal if not
carried out in the presence of the typical organics present
in aquaria. So when you see products make claims about their
ability to remove metals, be skeptical unless you understand
what conditions the claims relate to.
Input of Metals: Foods
If the goal is to reduce metals, then looking
at the foods that you feed can be important. It will be impossible
to eliminate all additions of metals this way, because all
marine-sourced materials contain significant amounts of metals
that they absorbed when growing in the ocean. Some of these
metals are used by the organisms involved, and some are just
incidentally accumulated. Nevertheless, there are some things
that you might consider when selecting foods if you want low
metals.
It turns out that there has been a fair
amount of study of many foods for certain metals because they
impact human health in various ways. For healthy people looking
to ingest adequate copper levels in food, the USDA recommends
shellfish, among other things, because they have a naturally
high level of copper and zinc.7,8
Some fish and shellfish may also be unusually high in many
metals (including copper, zinc, cadmium, mercury, and lead)
because of local pollution in the areas where they are harvested.9,10
People with Wilson's disease have problems
dealing with elevated copper levels, and they need to restrict
dietary copper. At a website
designed for people with this condition is a table
listing the copper levels in many foods, including many that
we feed to our aquaria (Table 1).
Table 1. The copper concentrations present in certain
shellfish.
|
Food
|
Copper
Concentration (ppm wet)
|
|
Fish
|
0.61
|
|
Scallops
|
0.27
|
|
Clams
|
6.1
|
|
Crab
|
7.4
|
|
Shrimp
|
1.8
|
|
Oysters
|
2.9
|
|
Mussels
|
4.8
|
|
Lobster
|
37.0
|
From this table it is clear that one can
select lower copper foods when shopping at the grocery store.
Scallops and shrimp, for example, would be much better choices
than clams, crab, or lobster. Also, the viscera of squid and
crabs contain much more in the way of heavy metals than does
muscle tissue.10 Over the
course of a year, these contributions really add up. If you
add 5 grams of each of the foods in Table 4 to a 100-gallon
aquarium every day, the addition over a year amounts to 178
ppb of copper using lobster and 1.3 ppb of copper using scallops.
For comparison, the amount of copper in the salt mixes described
in Shimek's article1 as
being high in metals are on the order of 100-200 ppb copper
(but only 18 ppb copper in an earlier article2),
and those low in copper were 1-40 ppb copper (for comparison,
my aquarium using only Instant Ocean salt mix presently has
10-13 ppb copper). So obviously, the choice of foods can potentially
make a big impact on the copper levels.
Many aquarists feed commercial foods to
their aquaria, rather than fresh seafood. In a study of the
amounts of different elements in certain foods,11
Shimek presented the results shown in Tables 2 and 3. While
none of these foods appears as high in copper as lobster,
lancefish is close and the differences between the various
foods are significant. In these tables I have highlighted
those values that stand out as unusually high in red and unusually
low in green. Bear in mind that some of these foods contain
substantial water, and so are naturally more "dilute".
For that reason, I included the first line in each table that
shows the calories/gram for each food. In this sense, it is
easy to see that the "wet" foods are about 4-5 times
less concentrated than the dry foods, so in looking at metals,
their concentrations need to be multiplied by 4-5 to get equivalent
values in terms of actual dosing.
Based on this metals analysis alone, and
no other nutritional properties, the Tahitian Blend would
seem to be a good overall choice if lower metals were a significant
goal (However, Eric Borneman has indicated that Tahitian Blend
is a plant material suspension that is of a particle size
that will be unusable by many organisms (pers.
comm.)). If we knew exactly what specific
metal to be most concerned with, the choice might well be
different.
Table 2. The metals content of a variety of commercial
foods (ppm as is).
|
Metals
|
Formula
One
|
Formula
Two
|
Prime
Reef
|
Lancefish
|
Brine
Shrimp
|
Plankton
|
|
Calories/gram
|
0.8
|
0.8
|
0.8
|
0.9
|
0.3
|
0.7
|
|
Aluminum
|
15.00
|
15.00
|
11.00
|
9.80
|
120.00
|
8.10
|
|
Arsenic
|
<0.50
|
<0.46
|
<0.52
|
2.10
|
<0.44
|
<0.42
|
|
Barium
|
0.55
|
0.73
|
0.72
|
<0.025
|
0.72
|
0.63
|
|
Cadmium
|
0.08
|
0.10
|
0.07
|
<0.02
|
<0.02
|
<0.02
|
|
Chromium
|
0.28
|
0.07
|
0.12
|
1.10
|
0.52
|
0.18
|
|
Cobalt
|
0.10
|
0.10
|
0.12
|
0.11
|
0.11
|
0.07
|
|
Copper
|
2.30
|
1.80
|
2.00
|
24.00
|
1.30
|
10.00
|
|
Manganese
|
4.40
|
13.00
|
14.00
|
3.40
|
10.00
|
0.62
|
|
Molybdenum
|
<0.25
|
<0.23
|
<0.26
|
<0.25
|
0.22
|
<0.21
|
|
Nickel
|
<0.25
|
<0.23
|
<0.26
|
<0.25
|
0.32
|
<0.21
|
|
Tin
|
0.72
|
0.70
|
0.70
|
2.40
|
0.34
|
0.38
|
|
Zinc
|
37.00
|
99.00
|
120.00
|
30.00
|
6.90
|
5.80
|
Table 3. The metals content of a variety of commercial
foods (ppm as is).
|
Metals
|
Gold
Flakes
|
Tahitian
Blend
|
Saltwater
Staple
|
Nori
|
Golden
Pearls
|
|
Calories/gram
|
4.2
|
2.4
|
3.6
|
3.6
|
3.9
|
|
Aluminum
|
80.00
|
14.00
|
95.00
|
83.00
|
49.00
|
|
Arsenic
|
2.30
|
<0.17
|
2.70
|
25.00
|
3.70
|
|
Barium
|
5.20
|
0.83
|
6.90
|
5.90
|
1.70
|
|
Cadmium
|
<0.84
|
<0.02
|
1.30
|
1.20
|
0.90
|
|
Chromium
|
5.60
|
0.80
|
<0.05
|
1.30
|
1.00
|
|
Cobalt
|
0.80
|
0.40
|
0.80
|
1.30
|
4.40
|
|
Copper
|
10.00
|
6.50
|
9.50
|
3.00
|
22.00
|
|
Manganese
|
23.00
|
18.00
|
90.00
|
110.00
|
49.00
|
|
Molybdenum
|
1.80
|
0.19
|
0.61
|
<3.30
|
1.20
|
|
Nickel
|
1.80
|
0.30
|
0.25
|
<3.30
|
<0.23
|
|
Tin
|
2.50
|
1.40
|
1.40
|
4.80
|
1.10
|
|
Zinc
|
63.00
|
12.00
|
190.00
|
38.00
|
280.00
|
Input of Metals: Limewater
Limewater (the English word for kalkwasser,
the solution that forms when either calcium oxide or calcium
hydroxide is dissolved in water) turns out to be a very useful
way to add calcium and alkalinity to a reef aquarium while
adding a minimal amount of certain heavy metals. This result
is not because the starting calcium hydroxide or oxide is
especially low in impurities, but because it can be self-purifying
if used correctly. It has been known for nearly 100 years,
for example, that copper is not soluble in limewater. According
to some folks from the US Department of Agriculture in 1908:
"The solutions obtained by adding
excess of lime to copper sulphate solutions of various concentrations
were found to be free from copper and were alkaline."12
They report that blue copper hydroxide
precipitates from the solution. Of course, analytical techniques
are much better now than they were in 1908, and the solutions
that they thought to be "free" of copper still contain
some dissolved copper at low levels, but much less than the
initial solutions. Since that time, lime has been used for
a variety of purposes relative to removal of copper from metal
etching solutions,13 wastewater,14
and sewage.15 It has also
been used to prevent the dissolution of copper from the insides
of pipes.16 The use of lime
to precipitate metals has also been shown for tin,17
and nickel.18
Why does this work and how can we use it?
It turns out that at high pH, many metals
form insoluble hydroxide and oxide salts. In fact, high pH
is a generally useful way to precipitate or limit the solubility
of many metals.19-23 If the limewater solution is allowed
to settle so that only clear limewater is dosed, then these
insoluble hydroxide salts will precipitate from solution and
collect on the bottom of the limewater reservoir. For example,
copper in the starting lime (or in the water used to make
it) will precipitate as copper hydroxide:
Cu++
+ 2OH- ŕ Cu(OH)2 solid
Consequently, as long as the solid precipitates
are not dosed to the aquarium, then the solution may well
be self-purifying for some of these metals. This has been
evidenced by the fact that some aquarists find blue precipitates
(perhaps copper hydroxide) remaining after they have used
limewater. Exactly how effective this process is at reducing
the concentration of metals depends on the metal, the pH,
and whether vinegar (and how much) has been used in the limewater
(vinegar alters the pH and hence the solubility of many hydroxide
species, it also potentially allows for soluble metal acetates
to exist in solution). Some metals can form soluble complexes
under the conditions present in limewater. Copper, for example,
can form the unusual species Cu(OH)3-.
It is the solubility of this species, rather than bare Cu++
that limits how effective limewater is at such self-purification.24
The calcium
oxide that I use from the Mississippi Lime Company is
food grade, but still has many impurities. Here's the typical
analysis as well as the required limits to food grade
CaO:
Table
4. Typical Chemical Analysis of Food Grade Calcium
Oxide
|
|
Si
|
0.35%
|
|
CaO
|
98.0%
|
|
LOI
|
0.50%
|
|
Magnesium
& Alkali Salts
|
1.0%
|
|
Fluoride
|
75
ppm
|
|
Lead
|
<0.5
ppm
|
|
Arsenic
|
<1.0
ppm
|
|
Acid
Insoluble Substances
|
0.20%
|
|
Heavy
Metals
|
2
ppm
|
|
Al
|
0.10%
|
|
Fe
|
0.04%
|
|
S
|
0.01%
|
|
CO2
|
0.40%
|
|
P
|
50
ppm
|
|
Mn
|
12
ppm
|
|
Ca
|
69.97%
|
|
Crystalline
Silica
|
<0.1
|
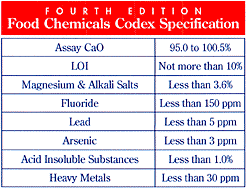
Notice that the typical analysis of the
calcium oxide that I use (Table 4) can contain 2 ppm heavy
metals, 0.1% aluminum, 12 ppm manganese, and less than 0.5
ppm lead. If one were
adding 2% of the aquarium volume in saturated limewater (0.0204
moles/L CaO) every day for a year, the following species would
be added to the water (Table 5).
Table 5. Cumulative amount of certain metals added
to a reef aquarium over the course of a year using limewater
if all of the impurities in the calcium oxide went into the
aquarium.
|
Metal
Species
|
Cumulative
Amount Added Per Year (ppb) [if
all goes in]
|
|
Calcium
|
5,956,000
|
|
Aluminum
|
8,300
|
|
Manganese
|
100
|
|
Total
Heavy Metals
|
16.7
|
|
Arsenic
|
<8
|
|
Lead
|
<4
|
Worse yet, the food grade specification
itself permits up to 15 times more heavy metals than the typical
analysis, for a whopping 250 ppb of total heavy metals and
60 ppb of lead additions per year. Depending on the nature
of those "heavy metals" those additions are potentially
quite significant compared to the 10-40 ppb of copper found
in many aquaria, and the much lower levels found in natural
seawater.
Unfortunately, not all metals will be removed
by precipitation as hydroxide or oxide salts. Aluminum, for
example, forms certain complexes in water that are soluble
at high pH. In future articles, I hope to show, either through
literature references or via experiment, how effective limewater
is at limiting the delivery of many of the metals that we
have been most interested in within this article.
Input of Metals: CaCO3/CO2
Reactors
The amounts of metals that are added to
an aquarium when using a CaCO3/CO2
reactor can also be determined. Unfortunately, such a system
is not "self-purifying", unlike limewater. Consequently,
most impurities in the CaCO3 media
make it into the aquarium water. The impurities present in
such media varies with the source or brand of the media, as
has been shown in different articles by Craig
Bingman25 and Greg
Hiller26. If we make
the assumption that we want the same total amount of calcium
and alkalinity as in the limewater case described above, then
we can calculate the following amounts of metals added over
a year: Those blocks colored red add more than the amount
detected in Shimek's study on Instant Ocean (IO) salt mix2
(many more should perhaps be colored red, but with none detected
in IO salt, it is not always possible to determine if it is
higher or lower than the IO salt mix).
Table 6. Cumulative amount of certain metals added
to a reef aquarium over the course
of a year using a CaCO3/CO2
reactor.
|
|
Conklin
Limestone
|
Nature's
Ocean
|
Koralith
|
Super
Calc Gold
|
|
Metal
|
Amount
added in 1 year (ppb)
|
Amount
added in 1 year (ppb)
|
Amount
added in 1 year (ppb)
|
Amount
added in 1 year (ppb)
|
|
aluminum
|
<1
|
1246
|
989
|
1060
|
|
arsenic
|
<1.7
|
<1.8
|
54
|
<22
|
|
barium
|
191
|
62
|
0
|
0
|
|
cadmium
|
<0.3
|
0.5
|
<5.0
|
<5.0
|
|
calcium
|
5956800
|
5956800
|
5956800
|
5956800
|
|
chromium
|
<0.3
|
44
|
<7
|
<7
|
|
cobalt
|
<0.3
|
1
|
10
|
20
|
|
copper
|
<1
|
4
|
96
|
88
|
|
iron
|
153
|
3830
|
0
|
940
|
|
lead
|
<1
|
<1
|
119
|
157
|
|
magnesium
|
62409
|
38876
|
14189
|
41785
|
|
manganese
|
1003
|
207
|
1003
|
1547
|
|
mercury
|
<1
|
<1
|
26
|
24
|
|
molybdenum
|
<0.3
|
<0.4
|
<6
|
<6
|
|
nickel
|
<0.3
|
4
|
192
|
227
|
|
phosphorus
|
904
|
2460
|
81
|
190
|
|
selenium
|
14
|
19
|
<81
|
<82
|
|
silicon
|
150
|
647
|
1125
|
1234
|
|
silver
|
<0.3
|
<0.4
|
14
|
<3
|
|
strontium
|
4774
|
9447
|
801
|
1492
|
|
sulfur
|
99
|
2027
|
28325
|
27210
|
|
tin
|
<17
|
<18
|
<46
|
<47
|
|
titanium
|
4
|
9
|
10
|
5
|
|
vanadium
|
<0.2
|
62
|
18
|
14
|
|
zinc
|
<0.2
|
<0.2
|
302
|
244
|
As can be seen, the amount added over the
course of a year can be quite substantial. In particular,
for several of the species of most interest from a toxicity
perspective (copper, zinc, nickel, aluminum, etc.) the amount
added is enough to take an aquarium from zero metals to more
than that present in a "high metal" salt mix, like
Instant Ocean.2
Input of Metals: Two-Part Calcium and Alkalinity
Supplements
When many aquarists first hear that the
two-part calcium and alkalinity additive systems (e.g., B-ionic,
C-balance, Kent Tech CD) contain copper and other metals,
their reaction is often quite negative. The claim of B-ionic,
for example, is that all ions present are in natural seawater
ratios except calcium and the bicarbonate and carbonate that
provide alkalinity. To fulfill that claim, copper and every
other metal must be present. Moreover, if they are truly present
at those levels, then they are not going to contribute to
elevated metals, but will, in fact, tend to actually reduce
them slightly over time.
Most aquarists realize that if a metal
such as copper is elevated in an aquarium, that a water change
with natural seawater will have a lowering effect on the concentration
of that metal. If these two-part additives are made exactly
as claimed, then the net effect of using them is the same
as a small water change with natural seawater (in addition
to supplementing calcium and alkalinity). For more skeptical
aquarists, I have shown how this works out mathematically
in a previous
article.
That said, I've seen no independent analyses
of any of these products. They may, in fact, not meet their
claim of having all ions in natural ratios. I hope to experimentally
test some of these products in the future.
Export of Metals: Macroalgae Growth and Harvesting
There are a number of potential ways to
export metals from reef aquaria. One that is already in use
by many aquarists, although perhaps not optimized for metal
export, is the growth and harvesting of macroalgae. There
have been a great many studies of the metals content of macroalgae
growing in the oceans of the world, and it has been found
that these algae accumulate metals to a significant degree.
Table 7, for example, summarizes some of the literature data.
Table 7. Literature values for the metals content of
certain macroalgae.
|
Metal(s)
Studied
|
Macroalgae
Studied
|
Concentration
(dry weight ppm)
|
|
Cu,
Pb and Zn
|
Twenty
six species of green, brown and red seaweeds27
|
Up
to 53 ppm
|
|
Cd
|
Twenty
six species of green, brown and red seaweeds27
|
1.6
|
|
Cr
|
Twenty
six species of green, brown and red seaweeds27
|
5.1
|
|
Co
|
Twenty
six species of green, brown and red seaweeds27
|
5.9
|
|
Mn
|
Various
species of green seaweed28
|
8-120
|
|
Mn
|
Various
species of brown seaweed28
|
29-125
|
|
Mn
|
Various
species of red seaweed28
|
13-160
|
|
Zn
|
Various
species of green seaweed28
|
14-36
|
|
Zn
|
Various
species of brown seaweed28
|
16-55
|
|
Zn
|
Various
species of red seaweed28
|
7-33
|
|
Cu
|
Various
species of green seaweed28
|
6-8
|
|
Cu
|
Various
species of brown seaweed28
|
6-19
|
|
Cu
|
Various
species of red seaweed28
|
5-29
|
|
Ni
|
Various
species of green seaweed28
|
0.6-5
|
|
Ni
|
Various
species of brown seaweed28
|
1.4-4
|
|
Ni
|
Various
species of green seaweed28
|
1.2-7
|
|
Co
|
Various
species of brown seaweed28
|
0.3-3
|
|
Co
|
Various
species of green seaweed28
|
0.5-1.3
|
|
Co
|
Various
species of brown seaweed28
|
0.4-4
|
|
Zn
|
One
species of Cyanophyta, 9 of Rhodophyta, 4 of Phaeophyta,
and 6 of Chlorophyta29
|
Some
above 100 ppm
|
|
Cu
|
One
species of Cyanophyta, 9 of Rhodophyta, 4 of Phaeophyta,
and 6 of Chlorophyta29
|
Some
above 20 ppm
|
|
Pb
|
One
species of Cyanophyta, 9 of Rhodophyta, 4 of Phaeophyta,
and 6 of Chlorophyta29
|
Some
above 10 ppm
|
As can be seen from this data, the amounts
of metals in these macroalgae is significant, and not very
different from that reported for nori and other foods earlier
in this article. They are also similar to the values reported
for Caulerpa sp. harvested from aquaria (~12 ppm dry
weight copper, 200 ppm dry weight zinc, 10 ppm dry weight
lead, etc.).30 If significant
amounts of these macroalgae are removed from an operating
reef aquarium, then the amount of export can be significant.
Depending on the type of food being used, and the extent of
macroalgae growth and export from the aquarium, the net export
of metals via macroalgae might even be more than the input
from foods.
Export of Metals: Activated Carbon
Activated carbon is known to bind copper
from freshwater,31 and it
is obviously known to bind organic materials in both marine
and freshwater aquaria. Since much of the copper present in
the water column of aquaria will be bound to organics, it
stands to reason that some of these organics will contain
copper. I've not seen any study of this effect, however, and
so the usefulness of activated carbon at metals export remains
unknown.
As a first pass, it should be relatively
easy to study by determining the copper concentration in a
sample of aquarium water (such as mine containing 10 ppb or
so of copper), exposing it to carbon, and redetermining the
copper concentration. In this experiment, copper is a good
test metal because it is largely bound by organics and so
should be a representative "best case" for metals
export via activated carbon. That experiment should at least
indicate if there is any potential use of carbon for aquarists
interested in low metals, and I hope to report on it in a
future article.
Export of Metals: Skimming
None of the inorganic forms of any of the
metals that we are interested in will be attracted to an air/water
interface. In fact, because of their extreme hydrophilicity,
they are generally repelled by it. Consequently, these forms
will not be appreciably removed by skimming.
Those metals that form complexes with organic
materials will, however, be removed by skimming as the organics
themselves will carry the metals out. It is difficult to say,
a priori, how effective skimming will be in such an
application. Some initial studies on skimmate show it to have
an appreciable metals content (including the metals of interest
to us),32 though in order
to determine how effective this process is for any given aquarium,
one must do careful balance studies on the skimmate and the
various sources of metals to any given aquarium.
Obviously, however, the more efficient
is the skimmer, the more likely is this pathway to be important.
Aside from boosting the skimming with a more powerful skimmer,
some folks have considered whether certain organic materials
can be added to an aquarium for the specific purpose of binding
metals and subsequently being skimmed out. Many commercial
polymers, for example, will likely function in this context.
Polyethyleneimine, for example, is shown complexing copper
in Figure 2. The concern with any material added for this
purpose is toxicity. Without knowing whether the material
to be added is safe for EVERY organism in the aquarium, it
is not possible to know whether it is worth the trade off
of reduced metals for a different potential toxin.
Figure 2. Copper ion complexed to the
nitrogen atoms in polyethyleneimine.
Export of Metals: Poly-Filters
Many aquarists have heard that Poly-Filters
(made by Poly-Bio-Marine)
absorb copper and other heavy metals. Poly-Filters are essentially
comprised of an organic polymer that is designed to bind to
a wide variety of chemical compounds in aquaria. As with all
materials that bind metals, the higher the concentration of
metal in solution, the more metal will be bound. Unfortunately,
this fact has lead many aquarists to misunderstand whether
Poly-Filters might actually help them reduce metal levels
below that present in typical reef aquaria (10-40 ppb). In
this section I am not discussing whether one can make a polymer
that will bind copper and other heavy metals from aquarium
water (that's a different discussion for elsewhere in this
paper and others, but it is possible that modified Poly-Filters
might work in that context). What we are interested here is
in whether there is any information to suggest that currently
available Poly-Filters are effective at reducing the copper
concentrations below the 10-40 ppb copper reported for all
of the marine aquaria in Shimek's study,
and in my own aquarium (10-13 ppb copper).
It turns out that, unlike most manufacturers,
Poly-Bio-Marine provides some nice
data and makes the results especially clear for us. Unfortunately,
what they say is that it won't work for many metals in artificial
seawater. In fact, they have specifically designed these filters
to not take out copper below 30 ppb. Here's a series of quotes
from their website:
“ASTM Standard D 1141 lists only six (6)
trace elements which are : Barium (99.4 mg/L),
Manganese (34.0 mg/L),
Copper (30.8 mg/L), Zinc (9.6 mg/L), Lead (6.6 mg/L)
and Silver (0.49 mg/L).”
Note:
mg/L is the same as ppb (parts per billion)
Then they note:
"Our next section will go into details
of how Poly-Bio-Marine, Inc.'s special manufacturing process
prevents Poly-Filter from sorbing those trace elements and
other major or minor synthetic seasalt components."
"In order to make a Poly-Filter not
capable of sorbing trace elements we must first saturate each
Poly-Filter with the trace elements found in synthetic seawater."
"Upon completion Poly-Filter will
not sorb trace elements nor calcium, magnesium, strontium
or fluoride."
So they add the metals listed above to
the Poly-Filters during manufacturing in order to prevent
them from bringing down these metal concentrations when used
in aquaria. In reality, I don't know whether their statements
are accurate or not in relation to real aquaria, because all
of the tests were in freshwater and synthetic seawater, not
in aquaria where some of these metals (especially copper)
will be largely bound to organics. Nevertheless, taking their
claims at face value, one is forced to conclude that Poly-Filters
will not be generally useful in reducing metal concentrations
below the levels shown in Table 8 when used in raw artificial
seawater. In this case, only zinc appears to be at a level
such that Poly-Filters will remove substantial amounts from
aquaria.
It is entirely possible that these filters
will be more effective than described below at removing metals
when the metals are bound to organics in real aquaria. After
all, these filters claim to remove organics as well. However,
it is also possible that they won't be effective, and testing
them under actual reef aquarium conditions is something that
I hope to provide in future articles.
Table 8. Removal of Metals by Poly-Filters.
|
Metal
|
Lower Limit of Removal
(ppb)
|
Levels
Found in Aquaria (ppb)3
|
|
Barium
|
99
|
5-33
|
|
Manganese
|
34
|
None
Detected (less than 0.5 ppb)
|
|
Copper
|
31
|
18-38
|
|
Zinc
|
10
|
190-260
ppb
|
|
Lead
|
7
|
None
Detected (less than 10 ppb)
|
|
Silver
|
0.5
|
None
Detected (less than 10 ppb)
|
Export of Metals: Other Systems
There are a variety of other systems that
one might imagine for metals export from reef aquaria. Some
of these are briefly described below.
1. Binding of metals to inorganic
media, like calcium carbonate, iron oxide or hydroxide, alumina,
and clay (e.g., kaolin). In this application, these materials
would be added to some sort of filter, like a fluidized sand
bed or maybe even something like a CaCO3/CO2
reactor with no CO2. It is well known
that metals bind to many of these materials.33-44
Iron oxide, for example, has been used to remove copper and
lead from wastewater.33,34
The binding of copper has even been studied on many media
in seawater in the presence and absence of organic materials.35
Nevertheless, how effective these systems will be in a real
aquarium can only be determined through experimental means,
and no such results are presently available.
Interestingly, absorption of metals onto
growing calcium carbonate surfaces, such as coral skeletons,
may well be the primary export mechanism for metals in reef
aquaria. Besides the fact that it is known that such surfaces
absorb metals, it is one thing that is common to all reef
aquaria. The inputs and other export mechanisms vary substantially
between reef aquaria, but if this export mechanism is the
one that ultimately limits the concentrations of certain metals,
such as copper, then that might explain why so many disparate
aquaria have similar copper levels.2
Likewise, if this export mechanism is so important and widespread,
then it may also be one that can be manipulated to lower the
metals content. Hence the suggestion of using calcium carbonate
media to bind and remove metals.
2. Polymeric resins specifically
designed to bind metals. I've patented a number of such materials
for human pharmaceutical use (to bind iron, copper, etc),
and there are vast numbers of other similar applications that
have been documented. Suitable materials would be those that
strongly complex metals, and could contain any of the many
organic functional groups know to interact strongly with the
metals of interest, including amines, hydroxamic acids, hydroxylamines,
carboxylic acids, phenols, thiols, and disulfides. These materials
would look and act rather like a Poly-Filter. In fact, Poly-Filters
provided without any metals content might even fit the need.
How effective these would be in a real aquarium remains to
be established. Some are commercially available and I hope
to test some in aquarium water.
The nature of the metal speciation will
greatly impact how well these materials work, and they might
work much more effectively in the environment of raw artificial
seawater than they will in a real aquarium environment, where
chelation by organics is important. Materials can be devised
that will bind the metals very strongly and can theoretically
take them away from the organic material to which they are
initially bound, but how fast that will happen, how much competition
there might be from ions that we do not want to bind (calcium,
magnesium, etc) and how much it might cost to do so leaves
this option uncertain.
Some materials of this nature are available
now, including the "Toxic Metal Sponge" by Kent
Marine and Cuprisorb by Seachem. How well these will work
at lowering the metals present in typical operating aquaria
to levels approaching natural seawater remains to be established.
3. Earlier in this article I mentioned
that many aquaria kept under different conditions with different
types of salt water (both natural seawater and artificial
saltwater) often seem to end up with similar levels of metals.
Copper, for example, seems to range between 5 and 40 ppb for
many aquaria. Why would that be? Certainly, binding to calcium
carbonate surfaces, as described above, might be the controlling
factor in driving all of these different aquaria to similar
copper levels.
Another possibility, however, is biological
control. Copper is known to bind to biological surfaces, ranging
from bacteria to corals. Millero shows a graph of copper binding
to bacteria, for example, and it demonstrates that the copper
binding is a steep function of the copper concentration in
the 5-50 ppb range.4 At
50 ppb there is about four times as much copper bound as at
5 ppb. Above 50 ppb (to about 200 ppb where the data ends)
there is no additional binding. Because of this copper binding,
as the dissolved copper concentration begins to rise within
the range of 5-50 ppb, copper will bind to bacteria and blunt
the observed rise. Likewise, as the copper concentration falls,
copper may be released and blunt the observed drop. Consequently,
the copper concentration may be "buffered" by binding
to bacteria and other biological surfaces in the range that
is typically found in aquaria. This binding may explain why
so many aquaria have similar dissolved copper levels.
While it is not easy to imagine using this
fact to lower concentrations below about 5 ppb (where little
binding occurs), it is clear that this pool of biologically
bound copper may make lowering the copper concentration in
aquaria substantially more difficult than it would be in a
tank that only contained water.
Summary
In this article I have provided background
information on the nature of metals in reef aquaria in order
to assist aquarists in their goals of understanding their
aquaria, and potentially learning to operate them with low
metals content. Whether this second goal ends up being worthwhile
will only be known when the fruits (if any) of such efforts
become generally recognized.
There are many sources of metals in aquaria,
perhaps many that are not mentioned here. Reducing this input
is one obvious way to try to reduce the metals content of
aquaria. In particular, selecting foods and calcium and alkalinity
supplements with low metals contents will be key to achieving
this goal.
Increasing the export of metals is the
other obvious way to achieve this goal. Some combination of
activated carbon, skimming, specific metal-binding media of
various types, and export of biological tissues (macroalgae,
corals, etc) may all end up being part of the plan.
Both the reduction of imports and the increase
of exports will likely be necessary to achieve metals levels
approaching natural seawater. Along the way, however, aquarists
may have significant difficulty in attaining their goal. If
the metals in the water column of aquaria are "buffered"
by binding of the metals to biological and inorganic surfaces,
then there may have to be substantial changes in the total
burden of metals in the tank before significant changes are
observed in the water column.
In future articles I hope to provide additional
information on how well some of the more unusual methods described
in this article actually succeed.
In the meantime… Happy Reefing!
|

